Organic hydridized tetra-core platinum complex and preparation method thereof as well as application technology field of complex in antitumor medicament preparation
A technology of organic hybridization and platinum complexes, applied in platinum group organic compounds, platinum organic compounds, antitumor drugs, etc., to achieve excellent antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
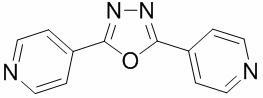
[0029] Synthesis of organic hybrid tetranuclear platinum complex with cisplatin as auxiliary ligand and bridging ligand of formula (III):
[0030] Formula (III)
[0031] Dissolve 0.3 millimoles of cisplatin in 6 milliliters of water, add 0.6 millimoles of silver nitrate in a dark place under the protection of nitrogen, and stir at 40°C for 15 hours. After the reaction is completed, centrifuge to discard the precipitate and keep the clear night; 0.3 mmol of bridging ligand (L 1 ), the entire reaction was reacted for 5 days at 60°C under the protection of nitrogen and protected from light. After the reaction, a large amount of absolute ethanol was added to the reaction solution to precipitate a pale yellow solid, which was centrifuged to obtain a pale yellow solid, and the product was dried in vacuum. Yield: 63%. Elemental analysis(%),
[0032] Theoretical value: C 32 H 48 N 24 O 24 Pt 4 ·5H 2 O (2021.24): C, 19.00; H, 2.89; N, 16.61. Experimental value: C, 19.36; H, 3.08; N, 16.53....
Embodiment 2
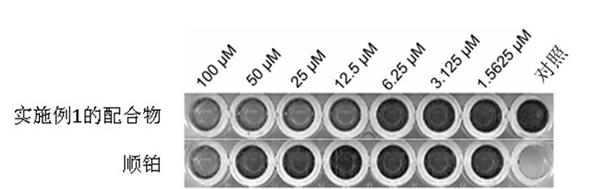
[0036] The anti-tumor activity test and results of the compound prepared in Example 1.
[0037] Cell lines and culture conditions: HepG2 (human liver cancer cell line), Hela (human cervical cancer cell line), MCF-7 (human breast cancer cell line), A549 (human lung cancer cell), A549 / cisR (cisplatin-resistant lung cancer cell) ) Provided by the Laboratory and Animal Center of Sun Yat-sen University. The cells were cultured in DMEM medium containing 10% fetal bovine serum, which contained 100 units of penicillin and 100 micrograms of streptomycin per milliliter. After the cells were inoculated in a petri dish with a diameter of 10 cm, 37 degrees, 5% CO 2 Cultivation in the environment, when the cells are full, trypsinization is used for passage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com