Novel human leukocyte antigen (HLA)-A2 limiting epitope polypeptide and use thereof
An HLA-A2, epitope peptide technology, applied in the fields of biology and medicine, can solve the problems of structural and functional influence, polypeptide inactivation, amino acid modification and modification methods and combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
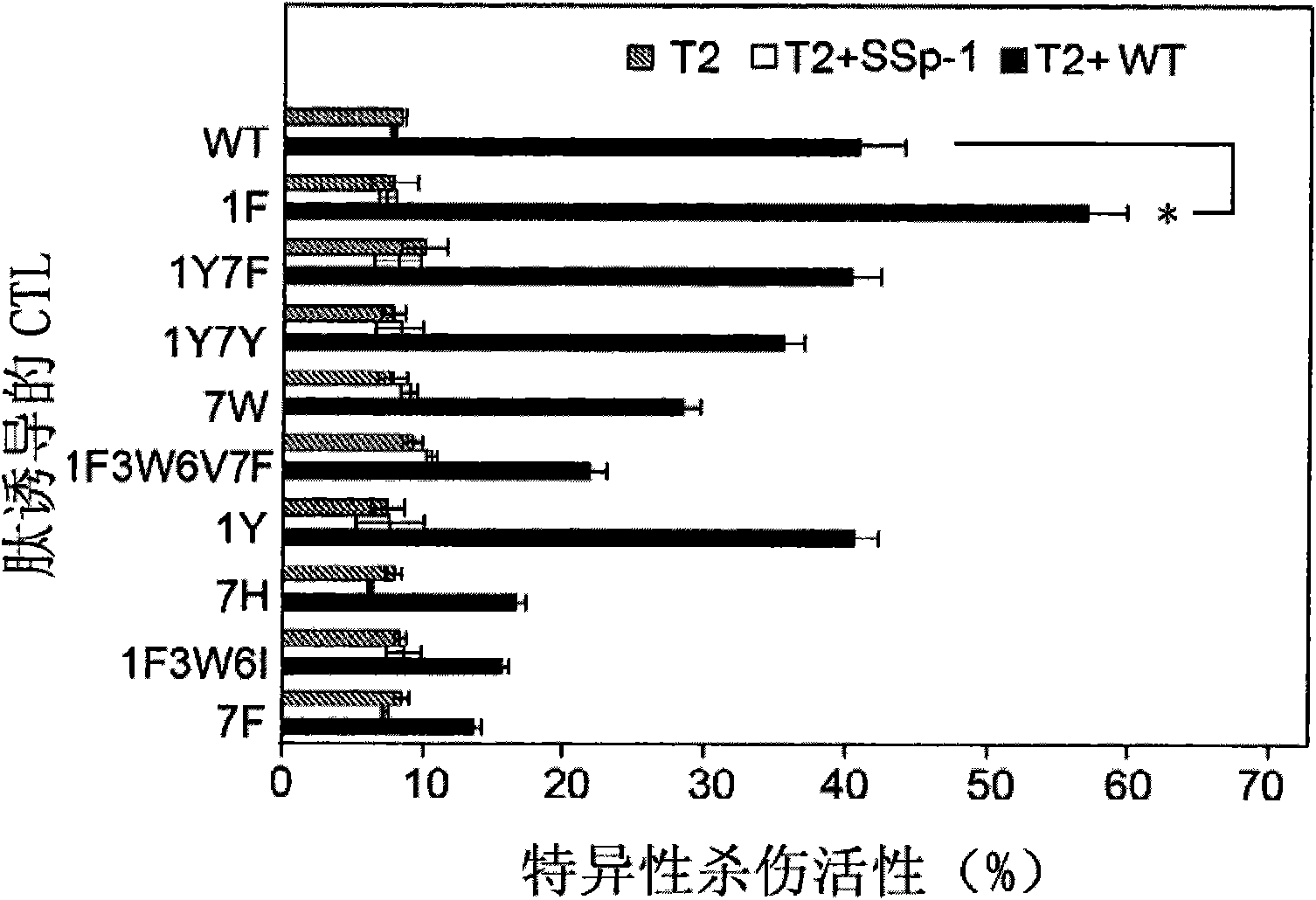
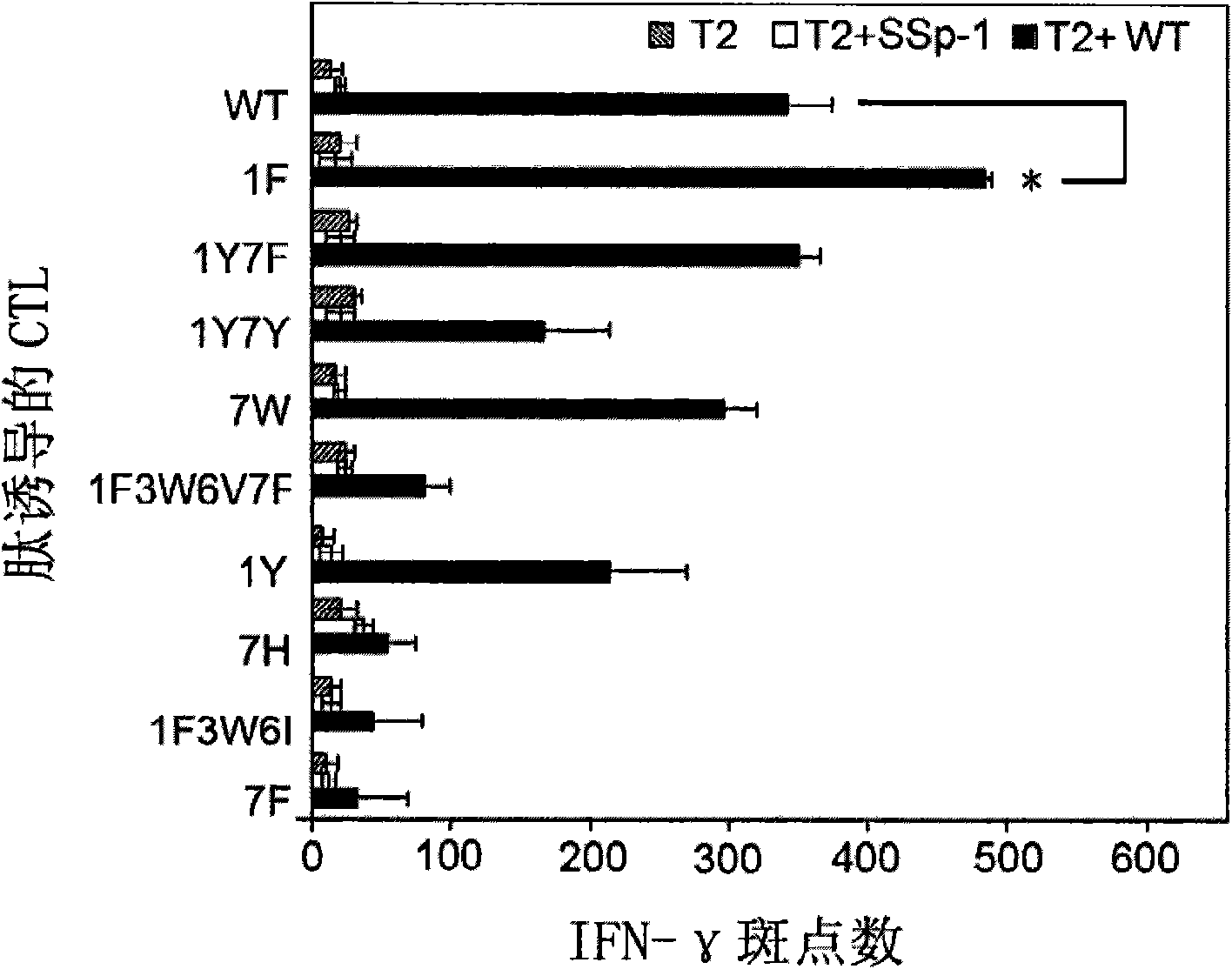
[0059] Example 1. Screening of HLA-A*0201 high-affinity peptides
[0060] In this example, epitope peptides with high affinity to HLA-A*0201 were screened by peptide binding experiments.
[0061] experiment procedure
[0062] First, T2 cells (a type of cells lacking antigen processing ability, purchased from ATCC: CRL-1991) were collected, washed three times with serum-free 1640 medium (purchased from Invitrogen), and the cell concentration was adjusted to 2 × 10 5 A / ml, spread in 24-well plate, 0.5ml / well. Then, add 50 μM candidate polypeptide, 2.5 μg / ml β2 microglobulin (purchased from R&D company) at 37°C, 5% CO 2 Incubate for 18h in total. After incubation, the cells were washed three times with ice PBS (pH 7.2, PBS was a buffer, self-prepared), and FITC-labeled HLA-A2-specific mAb BB7.2 (Sterotec Ltd, Oxford, UK) was added, and the ice bath was 45 After washing with PBS, the mean fluorescence intensity was detected by a flow cytometer (FACSCalibur, Beckton Dickinson...
Embodiment 2
[0073] Example 2. HLA-A2.1 / K b Induction of hPEBP4-derived HLA-A2-restricted polypeptide-specific cytotoxic T lymphocytes in transgenic mice
[0074] Preparation of effector cells
[0075] HLA-A2.1 / K was prepared according to conventional methods (refer to Inaba laboratory method, [Inaba, K. et al., J Exp Med. 1992; 176: 1693-1702]) b Bone marrow-derived dendritic cells (DC) from transgenic mice. Collect DCs cultured (refer to Inaba's laboratory method, ibid.) to day 7, and adjust the cell concentration to 1 × 10 6 cells / ml, add epitope peptides (each epitope peptide in Table 1 of Example 1, final concentration 10 μM / ml) and β2 microglobulin (final concentration 3 μg / ml), 37°C, 5% CO 2 Incubate in the incubator for 3h.
[0076] Collect peptide-sensitized DCs, wash three times with PBS (pH 7.2, PBS as buffer, self-prepared), and adjust the cell concentration to 1×10 6 each / 0.2ml (the amount of immunization is 0.2ml) immunized transgenic mice (HLA-A2.1 / K b Transgenic mic...
Embodiment 3
[0096] Example 3. Culture of human peripheral blood mononuclear cell-derived DCs sensitized with optimized epitope peptide 1F
[0097] Anticoagulated peripheral whole blood of HLA-A2.1+ / hPEBP4+ breast cancer patients was subjected to density gradient centrifugation (room temperature, 400 × g, 30 minutes) in lymphocyte separation medium (Ficoll-Histopaque 1.077, purchased from Sigma), and the interface cells were collected. Put it into a 50ml centrifuge tube, suspend the cells with calcium and magnesium-free PBS (pH7.2)-EDTA (2mM), and then wash the cells once by centrifugation (300×g, 10 minutes), discard the supernatant, and resuspend the cells in calcium and magnesium-free PBS. , and centrifuged again (200×g, 5 minutes) to wash the cells twice to obtain human peripheral blood mononuclear cells (PBMC).
[0098] Suspend PBMCs in complete medium (RPMI1640 with 10% fetal bovine serum) at 1×10 7 Cells / well were plated in 6-well plates at 37°C, 5% CO 2 After 2 hours of incubatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com