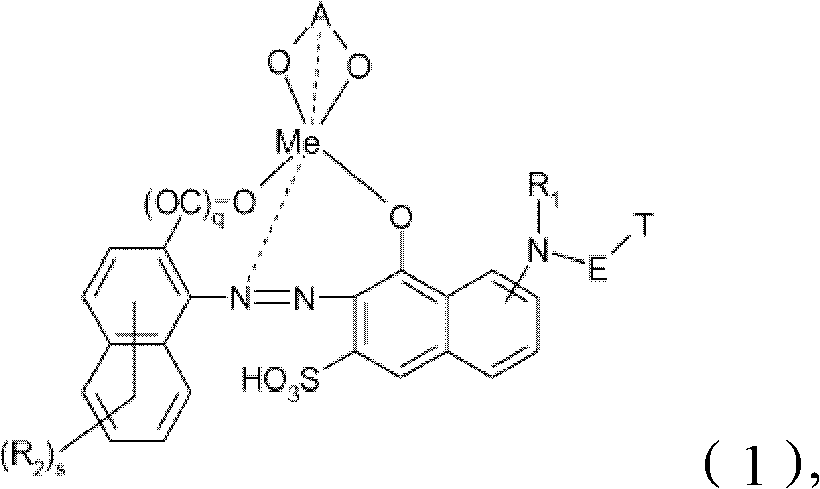
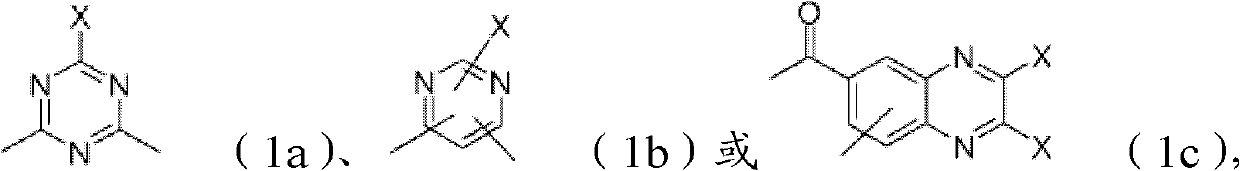Reactive dyes, their preparation and their use
A technology of reactive dyes and metal complex dyes, applied in the field of new reactive dyes, can solve the problems of unstable dyeing and unsatisfactory dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] (a) 26.4 parts of the free acid form are correspondingly compounds of the following structural formula
[0136]
[0137] Stir in 100 parts of water. The resulting slurry was cooled to 0°C by adding about 50 parts of ice and adjusted to pH 4 with aqueous sodium hydroxide solution, keeping its temperature at 0°C with ice. The resulting solution was slowly added to 18.2 parts of cyanuric chloride containing 20 parts of water, about 80 parts of ice and 0.2 parts of Na 2 HPO 4 12H 2 In the slurry in O, the pH was maintained at 3 by the addition of aqueous sodium hydroxide and the temperature was maintained at 0 °C by the addition of ice. A suspension is obtained comprising the free acid form of the compound of formula
[0138]
[0139] (b) 42 parts of free acid forms are correspondingly compounds of the following structural formula
[0140]
[0141] Slurry with 550 parts water, 15 parts formic acid and 6.8 parts chromium(III) acetate and heat in an autoclave at...
Embodiment 2
[0152] The dye according to example 1 was treated with sodium hydroxide at pH 11 for about 2 hours at room temperature. The resulting solution was freed from salt by dialysis and concentrated to dryness by evaporation. The dyestuff that obtains is free acid form and is correspondingly the compound of following structural formula and coordination isomer (λ 最大 =550nm)
[0153]
[0154] The dyes of formula (102) dye amino-containing fibers in Bordeaux shades with good permanent wet fastness properties even without postfixation, especially when the dyeing process described in EP-A 1687478 is used.
Embodiment 3-9
[0156] Dyes with the following structural formula
[0157]
[0158] (λ 最大 =480nm)
[0159]
[0160] (λ 最大 =499nm)
[0161]
[0162] (λ 最大 =462nm)
[0163]
[0164] (λ 最大 =459nm)
[0165]
[0166] (λ 最大 =461nm)
[0167]
[0168] (λ 最大 =574nm)
[0169]
[0170] (λ 最大 =570nm)
[0171]
[0172] (λ 最大 =573nm)
[0173]
[0174] (λ 最大 =570nm)
[0175]
[0176] (λ 最大 =577nm)
[0177]
[0178] (λ 最大 =459nm)
[0179]
[0180] (λ 最大 =552nm)
[0181]
[0182] (λ 最大 =552nm)
[0183]
[0184] (λ 最大 =588nm)
[0185]
[0186] (λ 最大 =606nm)
[0187]
[0188] (λ 最大 =568nm)
[0189]
[0190] (λ 最大 =570nm)
[0191]
[0192] (λ 最大 =584nm)
[0193]
[0194] (λ 最大 =464nm)
[0195]
[0196] (λ 最大 =572nm)
[0197] and the corresponding acrylate and vinyl ester forms, which can be prepared in a manner similar to that described in Examples 1 and 2 (except for the dyes of formulas (103) to (109) disclosed in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com