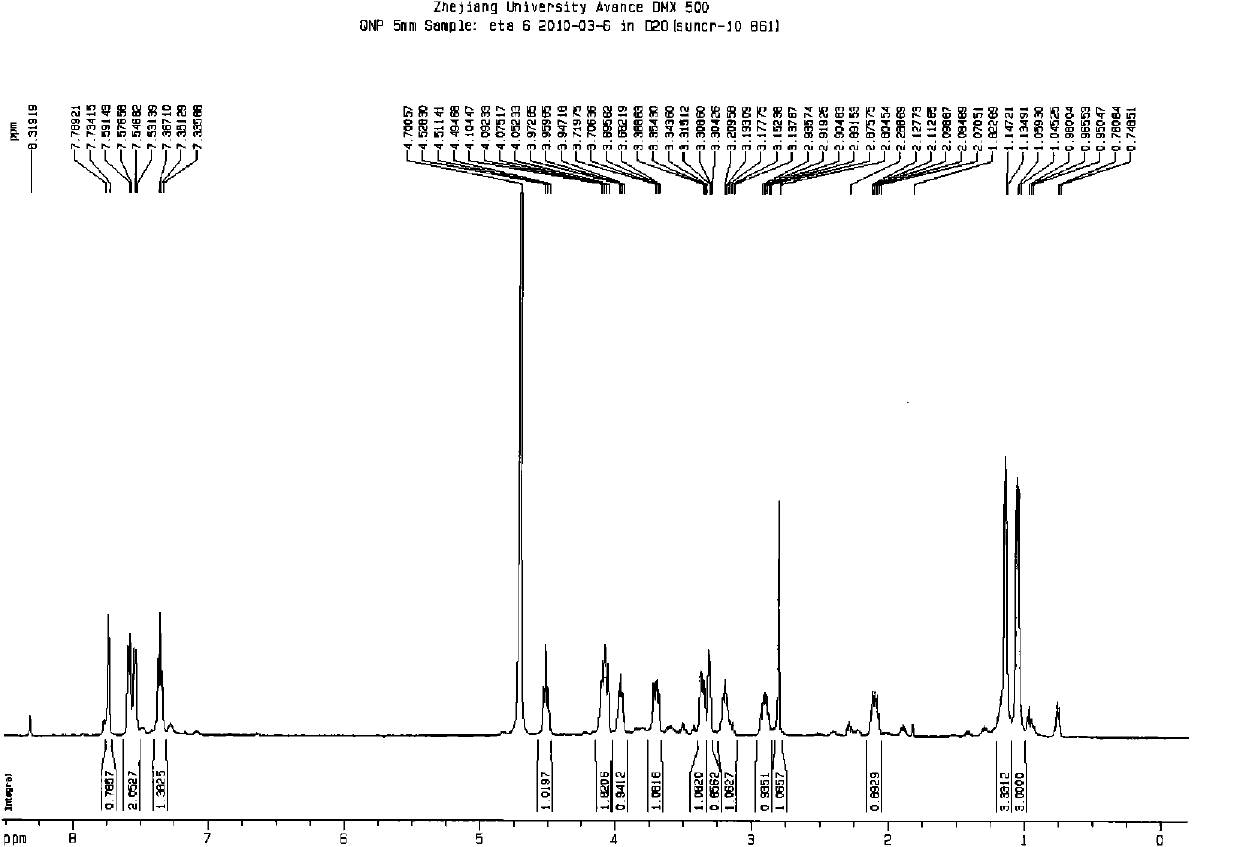
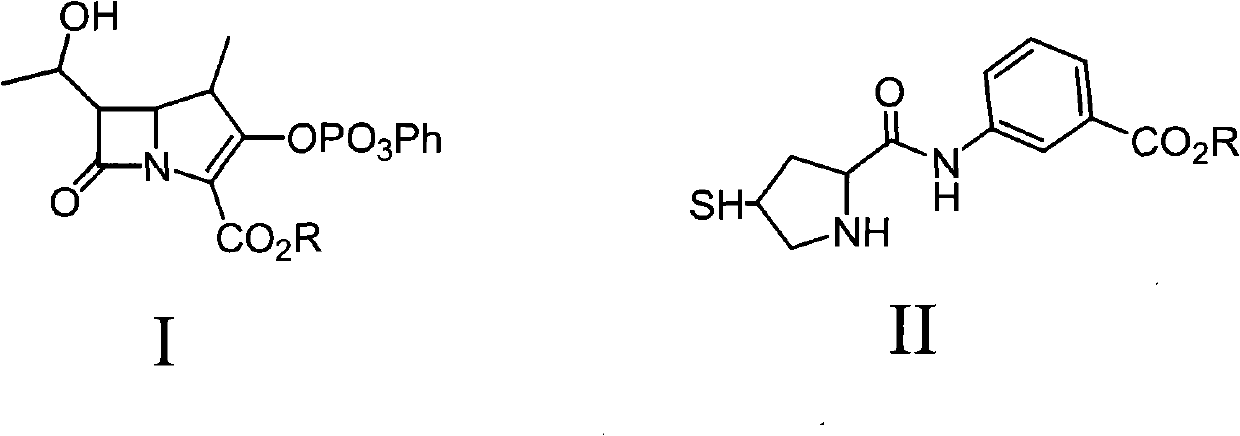
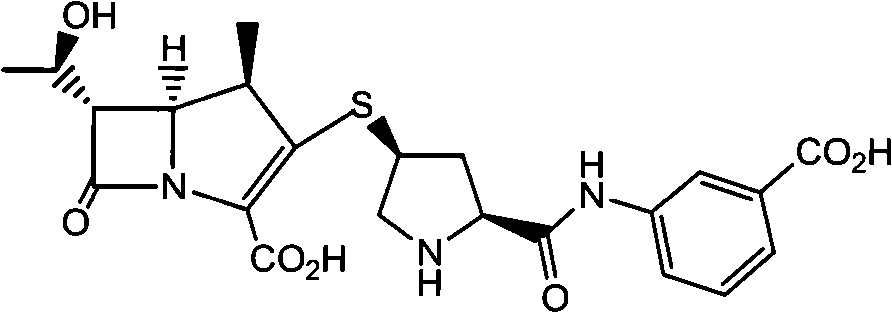
A kind of method that non-palladium system prepares ertapenem
A technology of ertapenem and parent nucleus, which is applied in the field of preparation of ertapenem, can solve the problems of difficulty in completely removing heavy metal palladium residues, potential safety hazards, cumbersome post-processing and the like, and achieves high conversion rate, low raw material cost, The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of giertapenem with protection from the mother nucleus (with MAP as the mother nucleus)
[0028]
[0029] Add 700ml of nitrogen ethylpyrrolidone (NEP) into a reaction flask equipped with a low-temperature thermometer and mechanical stirring, lower the temperature of the system to 0°C under nitrogen purge, add 85g of penem mother nucleus MAP, stir until dissolved and clarified, then add unprotected 42g of ertapenem side chain II, after stirring for a while, lower the temperature of the reaction system to -50°C, keep the temperature constant, quickly add 56g of diisopropylamine to the reaction system, fully stir the reaction at this temperature for about 5 minutes, and slowly raise the temperature The reaction was continued at -15°C for 10 minutes (the reaction was monitored by HPLC until the side chain disappeared), and immediately put into the next step of reduction hydrolysis reaction.
[0030] Preparation of Ertapenem
[0031]
[0032] Add the a...
Embodiment 2
[0034] Preparation of giertapenem with protected core (with MPP as the core)
[0035]
[0036] Add 600ml of acetonitrile into a reaction flask equipped with a low-temperature thermometer and mechanical stirring, lower the system temperature to 0°C under nitrogen purging, add 70g of penem mother nucleus MPP, stir until dissolved and clarified, then add unprotected ertapenem side chain II 42g, after stirring for a while, lower the temperature of the reaction system to -50°C, keep the temperature constant, quickly add 47g of diisopropylamine to the reaction system, and fully stir the reaction at this temperature for about 1 hour (HPLC monitors the reaction until the side chain disappears), Immediately put into the next step of reduction hydrolysis reaction.
[0037] Preparation of Ertapenem
[0038]
[0039] Add the above solution into the reaction flask, then add 2.7L of phosphate buffer (pH=5.0), 420g of zinc powder, and stir vigorously at 25°C for 3 hours under the ...
Embodiment 3
[0041] Preparation of Mother Nucleus and Side Chain Band Protected Giertapenem (with MAP as Mother Nucleus)
[0042]
[0043] Add 600ml of dichloromethane into a reaction flask equipped with a low-temperature thermometer and mechanical stirring, lower the system temperature to 0°C under nitrogen purging, add 85g of penem mother nucleus MAP, stir until dissolved and clarified, then add carboxyl-protected Ertape South side chain II 51g, after stirring for a while, lower the temperature of the reaction system to -50°C, keep the temperature constant, quickly add 47g of diisopropylamine to the reaction system, fully stir the reaction at this temperature for about 2 hours (HPLC monitors the reaction until the side chain disappear), immediately put into the next reduction hydrolysis reaction.
[0044] Preparation of Ertapenem
[0045]
[0046]Add the above solution into the reaction bottle, then add 2.7L of phosphate buffer (pH=6.0), 580g of zinc powder, and stir vigorousl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com