Bioelectrochemical sensor for detecting tumor markers and preparation method thereof
A tumor marker, bioelectrochemical technology, applied in the field of new bioelectrochemical sensors and their preparation, to achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
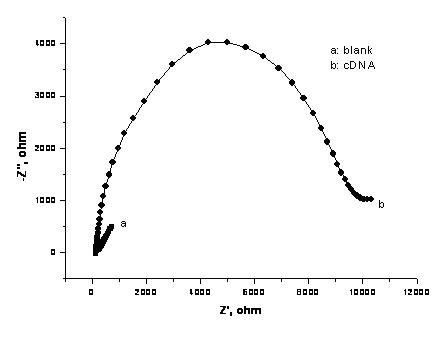
[0026] The gold electrode to be treated was sequentially polished on the silk containing alumina (grain size: 1 μm, 0.3 μm, 0.05 μm) mortar, then ultrasonically cleaned with ethanol and ultrapure water for 5 minutes, and then dripped on the surface of the gold electrode 10 μL piranha solution (H 2 o 2 : Concentrated H 2 SO 4 =3:1) for 2 minutes, wash away with ultrapure water, place the gold electrode in 0.5 M sulfuric acid solution, and perform cyclic voltammetry scanning in the voltage range of 0 - 1.6 V, with the scanning speed set at 100 mV / s , about 20 cycles to reach a stable state, and dry it with nitrogen gas to obtain a bare gold electrode with a clean surface, which can be used for the modification of sulfhydryl cDNA. The modification process is: immerse the cleaned gold electrode into the cDNA chain containing 1 μM sulfhydryl group (5'-SH-(CH 2 ) 6 -AGGAT CAACT GCGGC CAGCA CACCC AGATC CT-NH 2 -3') in an Eppendorf tube containing 10 mM Tris-HCl (pH 7.8), 1 mM E...
Embodiment 2
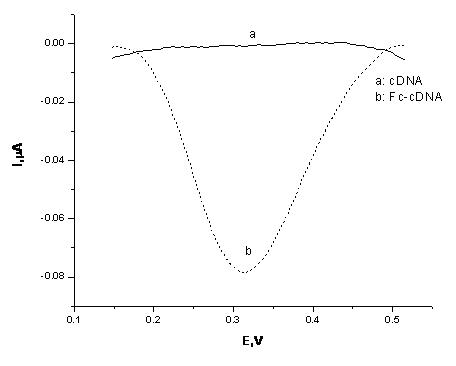
[0028]The gold electrode modified with the cDNA strand was immersed in a mixed solution of 5 mM EDC, 25 mM NHS, and 5 mM ferrocene carboxylic acid, and placed in a water bath at 37°C for 2 hours. During this process, the -NH at the 3' end of the cDNA strand on the gold electrode is modified 2 Condensation reaction occurs with -COOH of ferrocene carboxylic acid, so that Fc is attached to the end of cDNA chain. The result of this modification can be monitored by electrochemical method. like figure 2 In curve a, before Fc modification, because the cDNA chain cannot undergo redox reactions in the voltage range of 0 - 0.6 V, there is no obvious electrical signal; while curve b shows an obvious square wave voltage around 0.31 V An signal, which is generated by the Fc attached to the cDNA, indicates that the Fc molecule was successfully modified to the end of the cDNA chain.
[0029] Example 3: The sample contains only one tumor marker MUC1
Embodiment 3
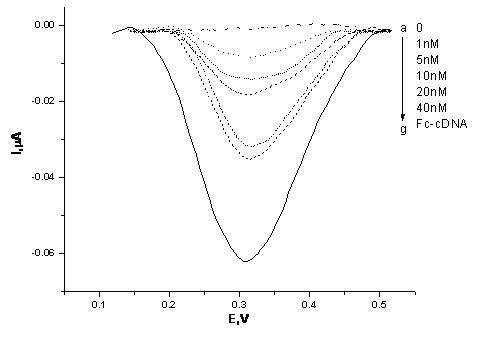
[0030] First, samples containing different concentrations of tumor marker MUC1 (1nM, 5nM, 10nM, 20nM, 40nM) were added to the test solution (10 nM MUC 1 aptamer, 10 nM VEGF 165 aptamer) for 1 hour at room temperature. During this process, part of the MUC1 aptamer in the test solution binds to its target protein, the tumor marker MUC1, and the rest of the MUC1 aptamer and all VEGF 165 The aptamer is freely dissociated in the test solution.
[0031] After the reaction, the gold electrode modified with Fc-cDNA was immersed in 100 μL of the above test solution, and reacted at room temperature for 1.5 hours to measure the electrochemical signal. The result is as image 3 As shown, with the increase of MUC1 concentration, the electrical signal gradually increased, and the reaction was basically saturated when the MUC1 concentration was 20 nM, and the electrical signal increased weakly when the MUC1 concentration was 40 nM. Therefore, we set 20 nM MUC1 as the optimal concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com