Vectors for the treatment of Alzheimer's disease
A carrier and carrier technology, applied in gene therapy, virus/phage, medical raw materials derived from virus/phage, etc., can solve the problems of unavailable vaccine technology and insufficient effect, so as to improve nursing care and improve the quality of life , the effect of reducing medical expenses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0204] [Example 1] Construction of SeV vector carrying Aβ42 gene
[0205] (1) Construction of the Not I fragment of the Aβ42 gene
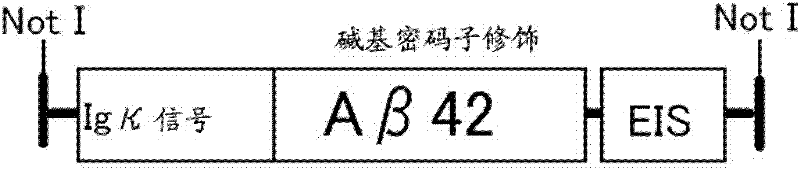
[0206] For the Aβ42 gene, assembly was performed by PCR using multiple primers covering the entire length of the human amyloid β peptide sequence (1-42) (SEQ ID NO: 1). The nucleotide sequence of Aβ42 was optimized considering human codon usage. The obtained sequence has the following structure: the Igκ secretion signal is bound to the N-terminal side, and the transcription signal of Sendai virus is added to the C-terminal side ( figure 1 , SEQ ID NO: 2).
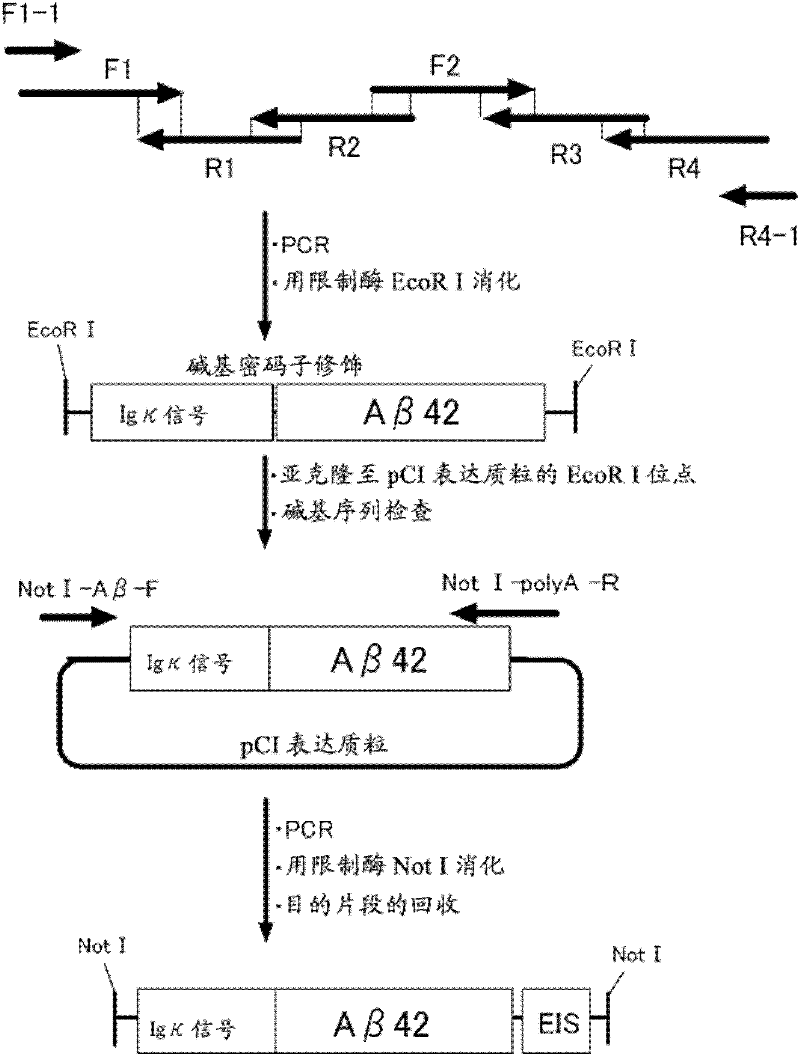
[0207] Build methods such as figure 2 shown. First, six long primers F1 (SEQ ID NO: 4), F2 (SEQ ID NO: 5), R1 (SEQ ID NO: 6), R2 (SEQ ID NO: 7 ), R3 (SEQ ID NO: 8), R4 (SEQ ID NO: 9) mixed. Using this primer compound, PCR was performed without adding a template. Then, using the PCR product as a template, PCR was further performed using two kinds of primers F1-1 (SEQ ID NO: 10) and R4-1 ...
Embodiment 2
[0211] [Example 2] Construction of a SeV vector carrying a fusion gene of Aβ42 and CTB (CTB-Aβ42)
[0212] (1) Construction of NotI fragment of CTB-Aβ42 gene
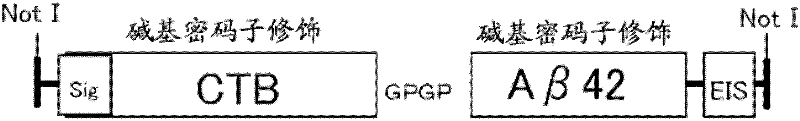
[0213] The NotI fragment of CTB-Aβ42 has the following structure: the N-terminal side of the sequence of the human amyloid β sequence (1-42) is linked to the cholera toxin B subunit sequence (SEQ ID NO: 14 ) connection, and the transcription signal of Sendai virus was added at its C-terminal side ( image 3 , SEQ ID NO: 15). Moreover, in order to increase expression efficiency, the nucleotide sequences of CTB and Aβ were changed according to human codon usage, but the amino acid sequences were not changed.
[0214] The gene was constructed by synthesizing the full length of the gene by performing PCR using multiple long primers covering the full length of the gene. Specifically, 8 kinds of long primers [CTB-AβF-1 (SEQ ID NO: 17), F-2 (SEQ ID NO: 18), F-3 (SEQ ID NO : 19), F-4 (SEQ ID NO: 20), R-1 (SEQ ID NO: 21), R-...
Embodiment 3
[0217] [Example 3] Construction of the SeV vector carrying the fusion gene of Aβ42 and IL-4
[0218] (1) Construction of the NotI fragment of the fusion gene of Aβ42 and IL-4
[0219] The fusion of Aβ42 gene and mouse IL-4 was carried out by partially overlapping and assembled by PCR.
[0220] Aβ42 gene utilization contains Aβ42 EcoRI fragment (embodiment 1: figure 2 ) plasmid preparation. Meanwhile, mouse IL-4 gene (SEQ ID NO: 27) was prepared as cDNA by the following procedure. mRNA was extracted from the spleen of mice (BALB / cA), reverse-transcribed using IL-4-specific primers, amplified by PCR, and subcloned into cloning plasmids. The resulting plasmid with mouse IL-4 cDNA as an insert was used for construction.
[0221]Specifically, using the mouse IL-4 plasmid as a template, two primers NotI-IL4-F (SEQ ID NO: 29) and IL4-R (SEQ ID NO: 30) were used for PCR. On the other hand, using the Aβ42 plasmid as a template, PCR was performed with primers Aβ42-F (SEQ ID NO: 31...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com