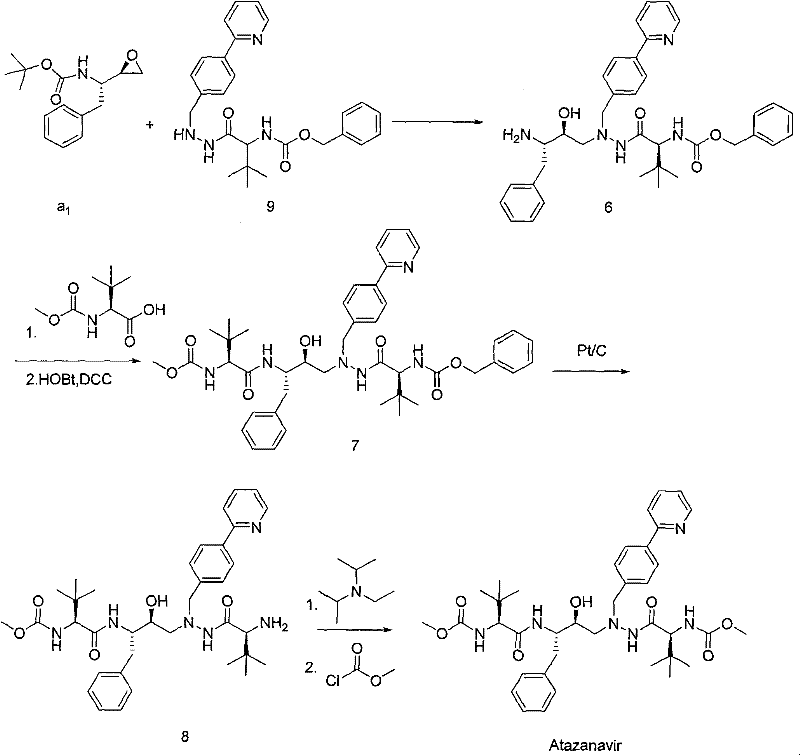
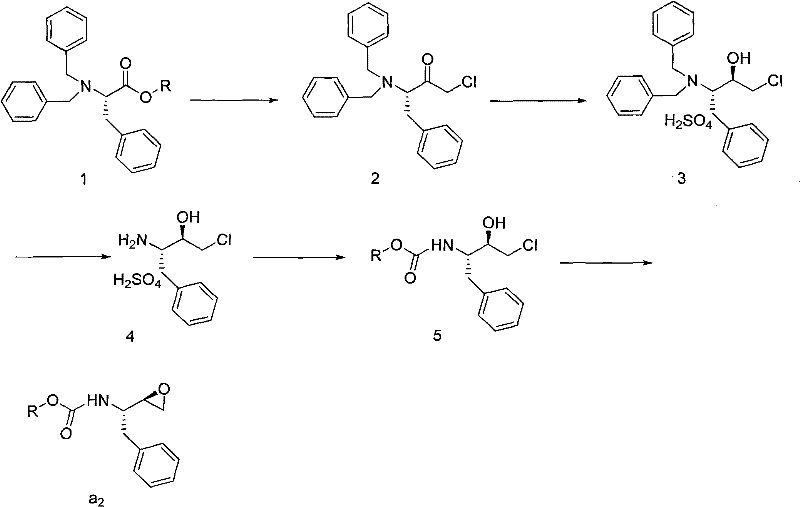
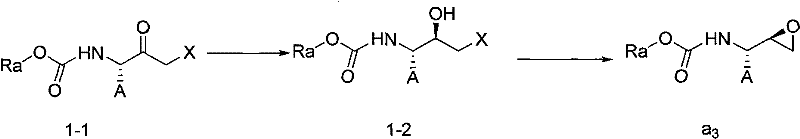
Preparation method of butylene oxide compounds and intermediates thereof
A compound and alkyl technology, applied in organic chemistry, bulk chemical production, etc., can solve the problems of sulfonyl halide compounds such as irritating odor, high cost, and high process cost, and achieve high industrial application and economic value. The effect of mild reaction conditions and simple reaction types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation of (4S, 5R)-4-benzyl-5-chloromethyl-2-oxazolidinone
[0056] Add (2S, 3S)-3-tert-butoxycarbonylamino-1-chloro-2-hydroxy-4-phenylbutane (10.0 g, 33.4 mmol) into 100 mL of anhydrous methyl tert-butyl ether, at 10 Slowly add SOCl dropwise at ℃ 2 (12.1 mL, 167.0 mmol), after the dropwise addition was completed, the reaction mixture was then heated to 50° C. and reacted at this reaction temperature for 8 hours. After the reaction was completed, cool to 0°C and wash with saturated NaHCO 3 The solution was quenched, separated, and the organic phase was concentrated to 100 mL to obtain a solution of (4S, 5R)-4-benzyl-5-chloromethyl-2-oxazolidinone in methyl tert-butyl ether with a concentration of 33.4 mol / L, used directly in the next reaction.
Embodiment 2
[0057] Example 2: Preparation of (4S, 5R)-4-benzyl-5-chloromethyl-3-tert-butoxycarbonyl-2-oxazolidinone
[0058] Take 50 mL of (4S, 5R)-4-benzyl-5-chloromethyl-2-oxazolidinone in methyl tert-butyl ether solution (50ml, 16.7mmol) obtained in the previous step reaction, add triethylamine (2.6mL, 18.0mmol), di-tert-butyl dicarbonate (3.64g, 16.7mmol) and 4-Dimethylaminopyridine (1.95g, 1.6mmol), the reaction mixture was stirred at 15°C for 8 hours. After the reaction was completed, 5.28 g of solid (4S,5R)-4-benzyl-5-chloromethyl-3-tert-butoxycarbonyl-2-oxazolidinone was obtained by filtration, with a yield of 97.1%.
[0059] 1 H NMR (500 MHz, CDCl 3 ( dd, J=11.9, 5.8 Hz, 1H), 3.35 (dd, J=13.5, 3.5 Hz, 1H), 3.29 (dd, J=11.9, 3.7 Hz, 1H), 2.84 (dd, J=13.5, 9.3 Hz , 1H), 1.60 (s, 9H). MS-ESI: 348.1 (M+Na + )
Embodiment 3
[0060] Example 3: Preparation of (2R, 3S)-3-tert-butoxycarbonylamino-1,2-epoxy-benzenebutane
[0061] (4S, 5R)-4-benzyl-5-chloromethyl-3-tert-butoxycarbonyl-2-oxazolidinone (2.0 g, 6.2 mmol) was added to 5 mL of ethanol, and potassium hydroxide ( 0.70g, 12.4mmol) was dissolved in 5mL ethanol, and this potassium hydroxide ethanol solution was slowly added dropwise to (4S, 5R)-4-benzyl-5-chloromethyl-3-tert-butoxycarbonyl-2- In the oxazolidinone ethanol solution system, after the dropwise addition, the reaction mixture continued to react at 20°C for 5 hours. After the reaction was completed, the solvent was removed under reduced pressure, and the residue was dissolved in 20 mL of ethyl acetate, extracted, and the organic phase was concentrated to obtain Color oily liquid (2R,3S)-3-tert-butoxycarbonylamino-1,2-epoxy-benzenebutane 1.56g, yield 95.2%, HPLC purity 99.3%, this oily liquid can be solidified at -20°C A white solid was obtained.
[0062] 1 H NMR (500 MHz, CDCl 3 ( ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com