Bis-nitroheterocyclic carbene bis-palladium complex and preparation method thereof
A technology of diazide heterocycle and palladium complex is applied in the field of double palladium complex and its preparation, can solve the problems of easy decomposition of synthesis steps, waste of silver and the like, and achieves the effects of simple synthesis method, high yield and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
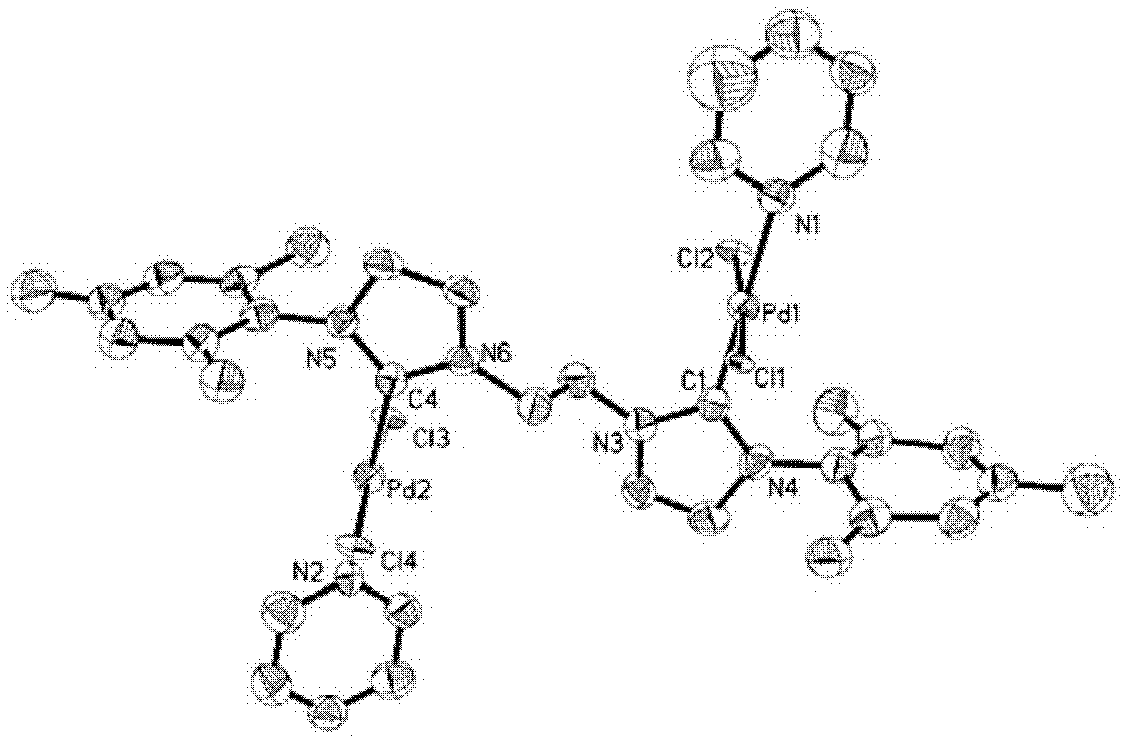
[0026] Example 1: L 1a (PdCl 2 ) 2 (Py) 2 Synthesis and Characterization of Binuclear Palladium Metal Compounds
[0027]
[0028] Add (L 1a ) 1,1'-di-trimethylphenyl-3,3'-(1,2-ethylene)diimidazole dichloride (1.414g, 3mmol), PdCl 2 (1.069g, 6.0mmol), K 2 CO 3 (8.293g, 60mmol), add a condenser and turn the cap on the condenser orifice. Ventilate the air three times on the vacuum line to ensure that the system is filled with argon. Use a syringe to take 35.5 mL of anhydrous treated pyridine and add it to the reactor. Insert a balloon filled with argon on the system opening plug and add it to the oil bath. Reaction at 85°C for 18h. After the reaction was completed, the reaction solution was passed through celite, washed with dichloromethane, and the filtrate was collected. The solvent was distilled off under reduced pressure to obtain a yellow crude product, 2.27 g of a light yellow solid recrystallized from dichloromethane / ether, with a yield of 83%.
[0029] 1 H N...
Embodiment 2
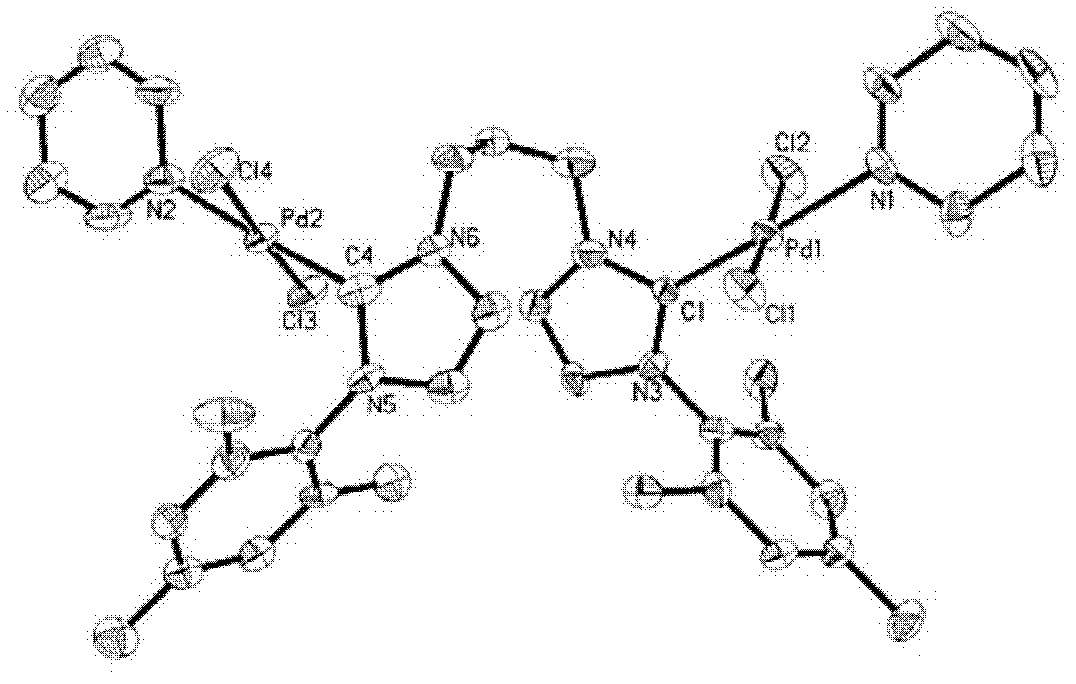
[0030] Example 2: L 1b (PdCl 2 ) 2 (Py) 2 Synthesis and Characterization of Binuclear Palladium Metal Compounds
[0031]
[0032] Add (L 1b ) 1,1'-di-me-trimethylphenyl-3,3'-(1,3-propylene) diimidazole dichloride (1.457g, 3mmol), PdCl 2(1.069g, 6.0mmol), and K 2 CO 3 (8.293g, 60mmol), add a condenser and turn the cap on the condenser orifice. Ventilate the air three times on the vacuum line to ensure that the system is filled with argon. Use a syringe to take 35.5 mL of anhydrous treated pyridine and add it to the reactor. Insert a balloon filled with argon on the system opening plug and add it to the oil bath. Reaction at 85°C for 18h. After the reaction was completed, the reaction solution was passed through celite, washed with dichloromethane, and the filtrate was collected. The solvent was distilled off under reduced pressure to obtain a yellow crude product, 2.142 g of a light yellow solid recrystallized from dichloromethane / ether, with a yield of 77%.
[003...
Embodiment 3
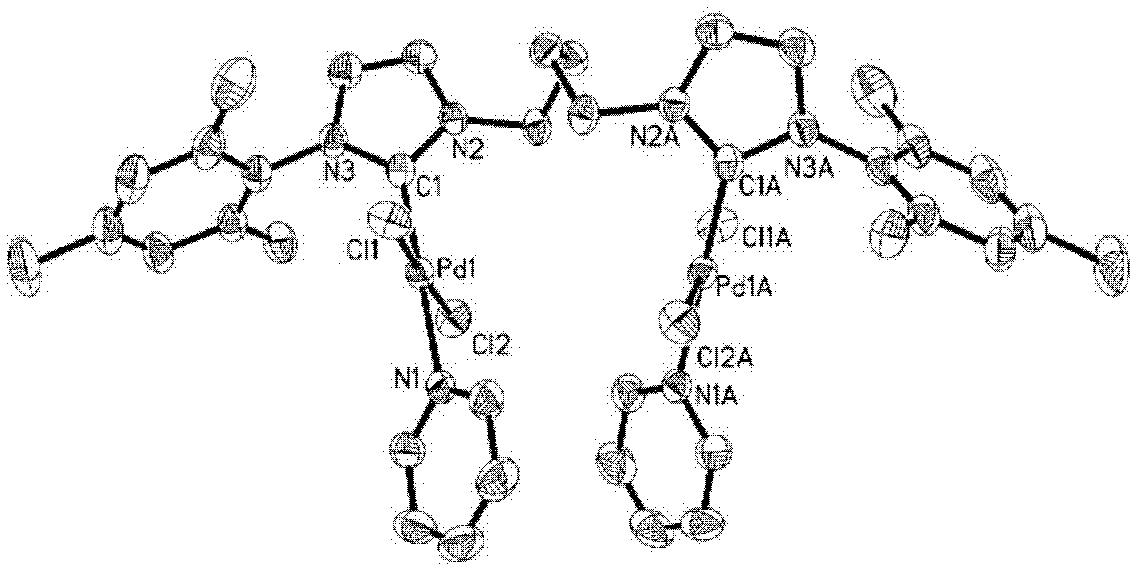
[0034] Example 3: L 1c (PdCl 2 ) 2 (Py) 2 Synthesis and Characterization of Binuclear Palladium Metal Compounds
[0035]
[0036] Add (L 1c ) 1,1'-di-me-trimethylphenyl-3,3'-(1,4-butylene)diimidazole dichloride (1.499g, 3mmol), PdCl 2 (1.069g, 6.0mmol), and K 2 CO 3 (8.293g, 60mmol), add a condenser and turn the cap on the condenser orifice. Ventilate the air three times on the vacuum line to ensure that the system is filled with argon. Use a syringe to take 35.5 mL of anhydrous treated pyridine and add it to the reactor. Insert a balloon filled with argon on the system opening plug and add it to the oil bath. Reaction at 85°C for 18h. After the reaction was completed, the reaction solution was passed through celite, washed with dichloromethane, and the filtrate was collected. The solvent was distilled off under reduced pressure to obtain a yellow crude product, 1.807 g of a pale yellow solid recrystallized from dichloromethane / ether, with a yield of 64%.
[0037]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com