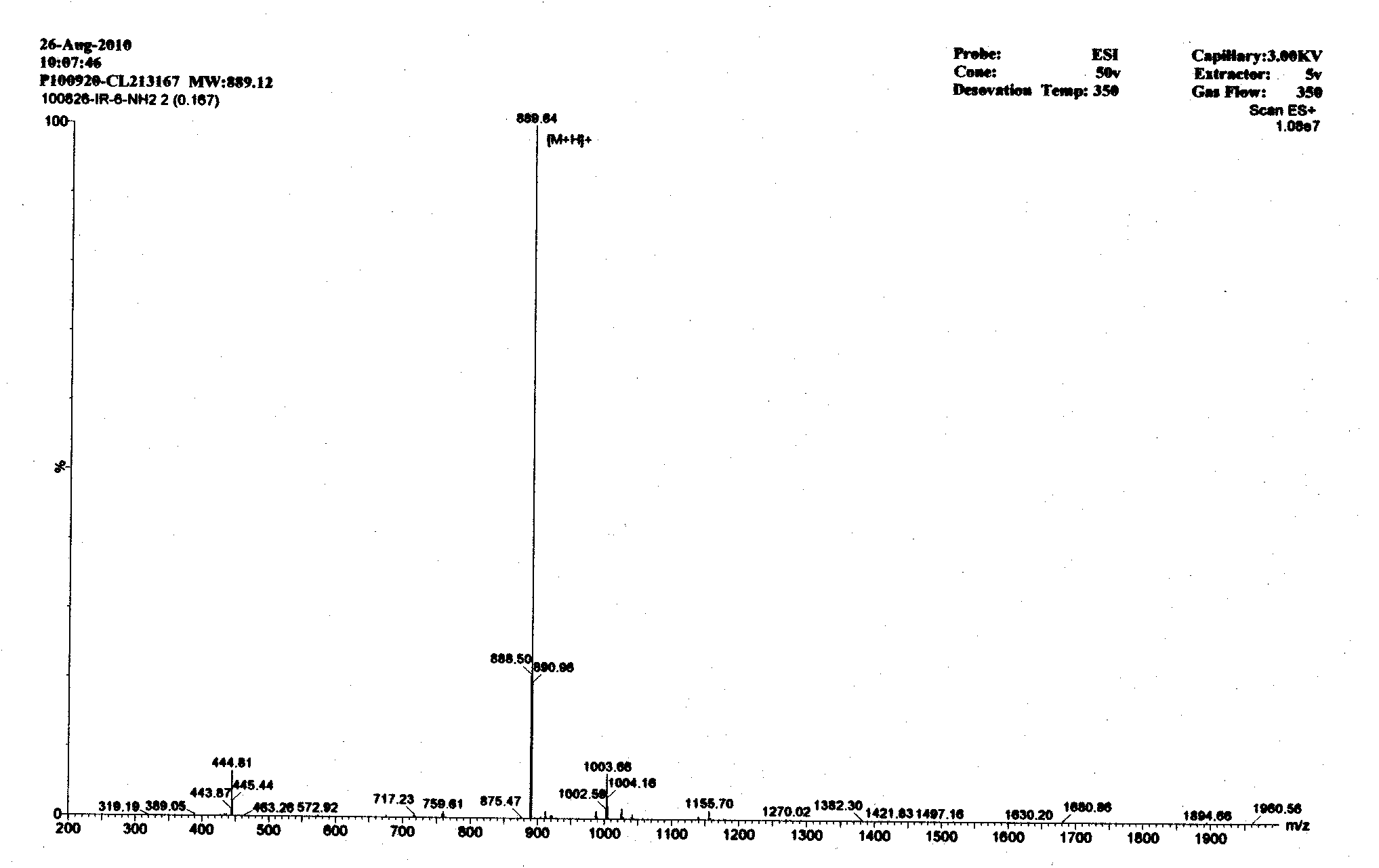
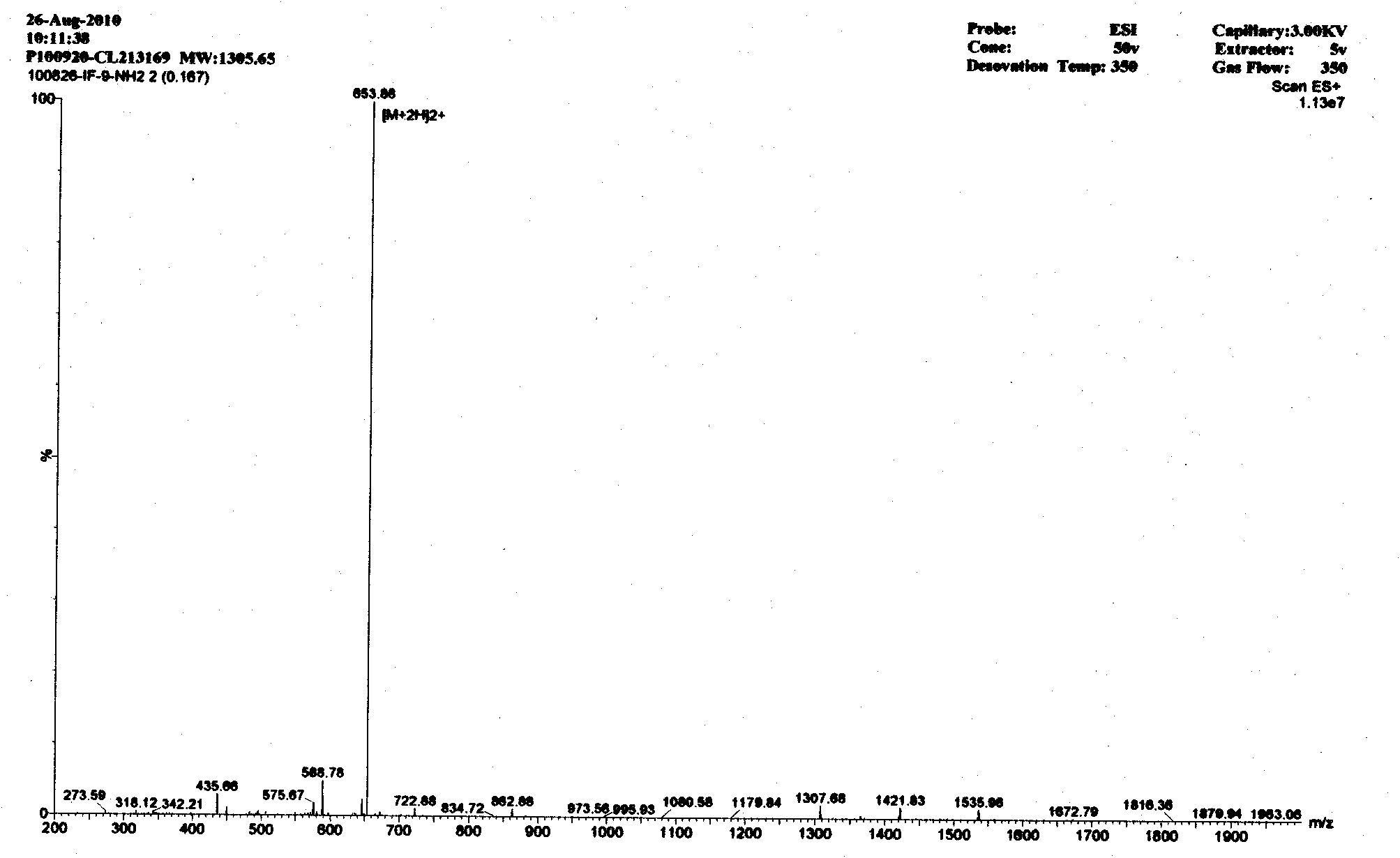
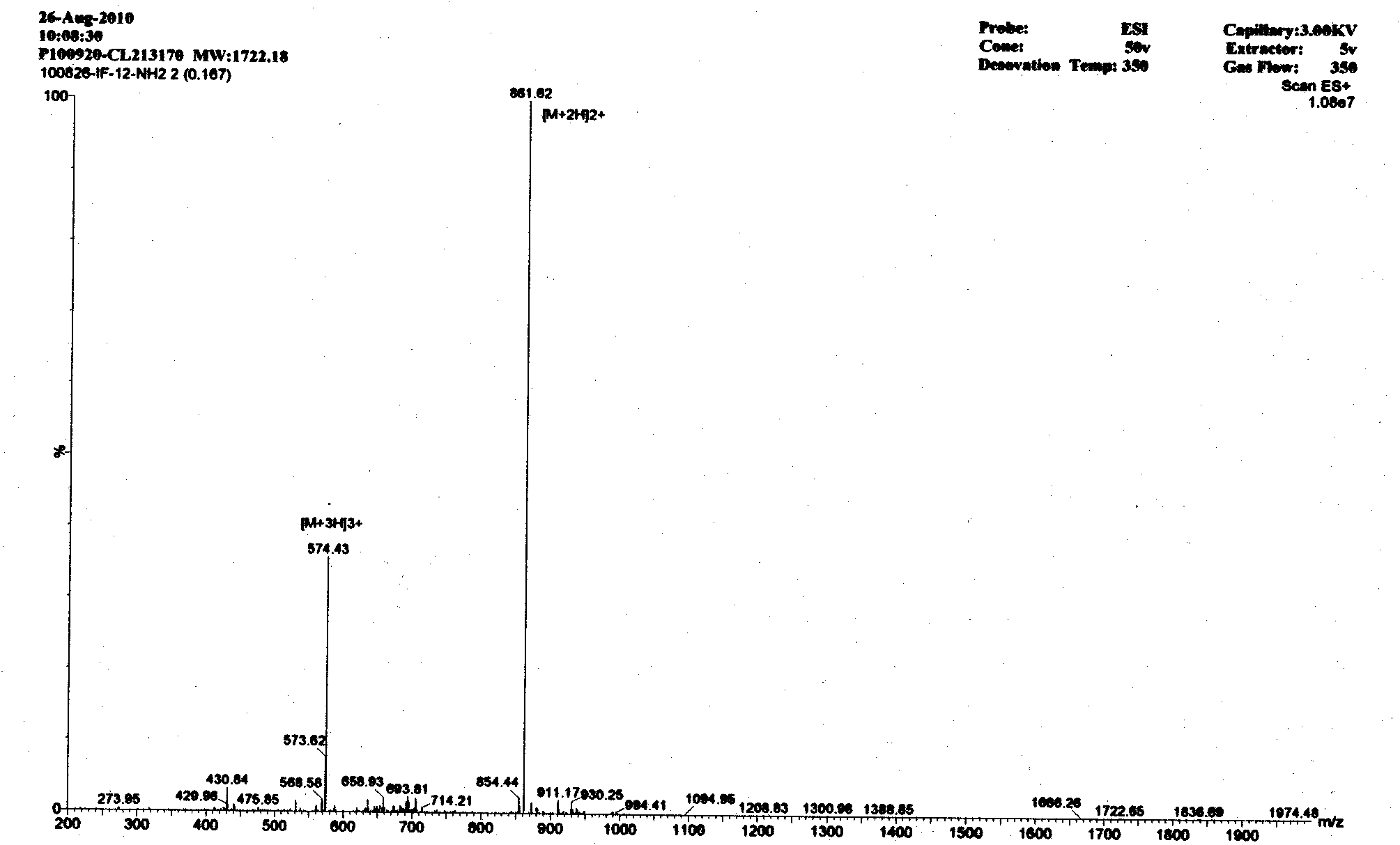
A kind of antibacterial peptide and preparation method thereof
A technology of antibacterial peptides and amino acids, applied in the field of artificially synthesized new antibacterial peptides and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] According to the mechanism of action of antimicrobial peptides and the characteristics of amino acid composition, the basic amino acid arginine (Arg) residues are used to provide positive charges to attract the negative charges on the surface of bacteria, and the aliphatic amino acids isoleucine (Ile) and The aromatic amino acid phenylalanine (Phe) has a hydrophobic interaction with phospholipids, thereby enabling antimicrobial peptides to damage bacterial cell membranes. According to this principle, there are five antimicrobial peptides in this embodiment, namely A-E in Table 1;
Embodiment 2
[0016] The above five peptides were synthesized using a peptide synthesizer, using solid-phase organic synthesis, and the synthesis direction was carried out one by one from the C-terminal to the N-terminal, and the Fmoc protection synthesis method was adopted. The specific steps were as follows:
[0017] Select the Wang resin that has been linked to the first amino acid at the C-terminal, that is, Fmoc-A(trt)-Wang (9-fluorenylmethoxycarboxy-trimethyl-A, where A is the first amino acid at the C-terminal), use two Soak in methylformamide (DMF) for about 15 minutes to remove impurities; remove the Fmoc protection on the resin with DMF containing 20% piperidine, react for 20 minutes, and wash the resin until it is complete. The piperidine was washed away with DMF, and the remaining solid suspension was the deprotected A-Wang. The quality of A-Wang deprotection was checked with ptrintrione detection reagent.
[0018] Fmoc-B(trt)-OH (9-fluorenylmethoxycarboxy-trimethyl-B, B is t...
Embodiment 3
[0024] 1) Determination of antibacterial activity: Prepare the peptide into a certain storage solution for use. The minimum inhibitory concentrations of several antimicrobial peptides were determined by the broth microdilution method. Using 0.01% acetic acid (containing 0.2% BSA) as the diluent, a series of gradient antimicrobial peptide solutions were sequentially prepared using the double dilution method. Take 100 μl of the above solution and place it in a 96-well cell culture plate, then add an equal volume of the bacteria solution to be tested (~10 5 pc / ml) in each well. Positive controls (containing bacterial fluid but not antimicrobial peptides) and negative controls (neither bacterial fluid nor peptides) were set up. Incubate at a constant temperature of 37°C for 20 hours, and the minimum inhibitory concentration is the one where no turbidity is seen at the bottom of the well with the naked eye.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com