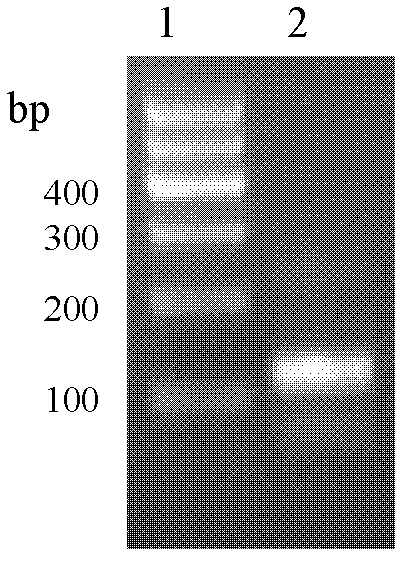
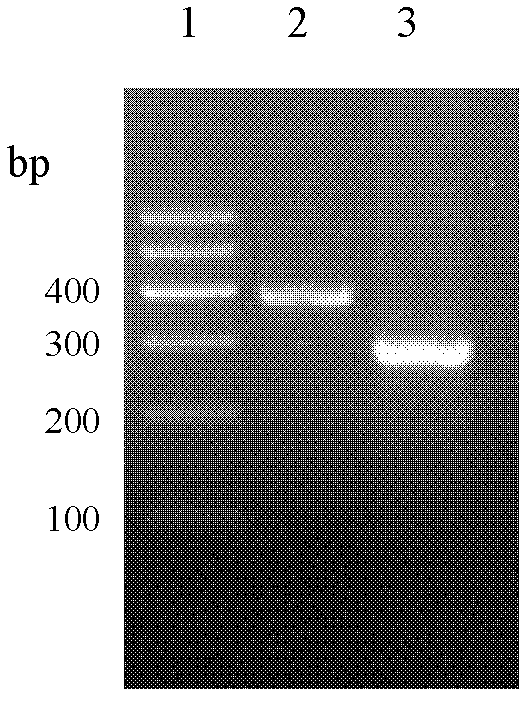
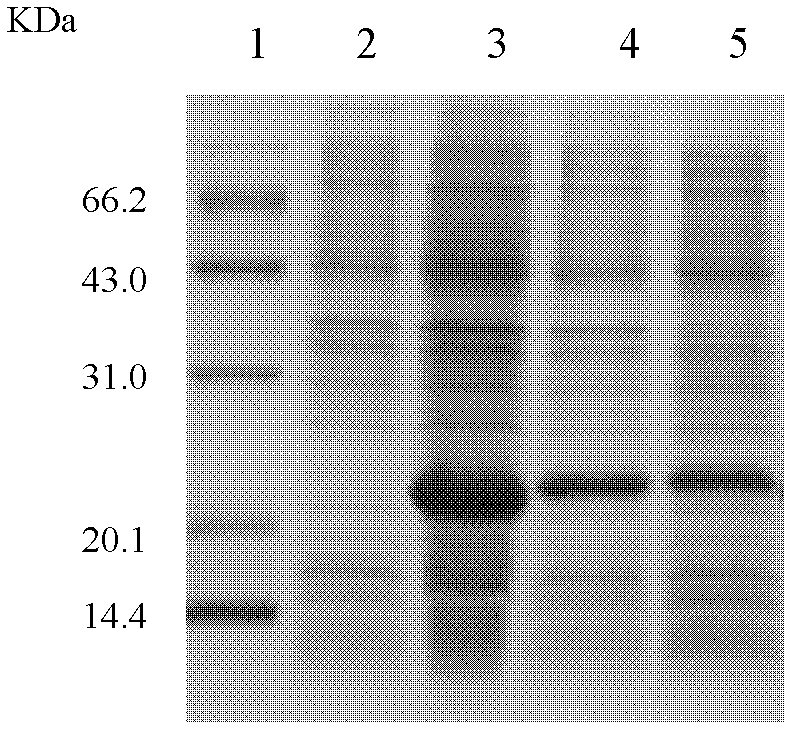
Antimicrobial peptide lf-th and its Escherichia coli recombinant preparation method
A technology of LF-TH and Escherichia coli, which is applied in biochemical equipment and methods, recombinant DNA technology, chemical instruments and methods, etc., can solve the problem of low antibacterial activity of antimicrobial peptides, achieve broad antibacterial spectrum, broad application potential, antibacterial The effect of activity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0015] 具体实施方式一:本实施方式抗菌肽LF-TH的氨基酸序列包括33个氨基酸,其氨基酸序列如下:PheLysCysArgArgTrpGlnTrpArgTrpLysLysLeuGlyAlaLysProValProIleIleTyrCysAsnArgArgThrGlyLysCysGlnArgMet,其编码基因序列如下:TTCAAATGCCGTCGTTGGCAGTGGCGTTGGAAAAAACTGGGTGCGAAACCGGTTCCGATCATCTACTGCAACCGTCGTACCGGTAAATGCCAGCGTATG。
specific Embodiment approach 2
[0016] Specific embodiment two: The Escherichia coli recombinant preparation method of the antimicrobial peptide LF-TH in this embodiment is carried out according to the following steps:
[0017] 1. Select the 1st to 15th amino acid sequence Lfcin B (1 to 15) of LfcinB, wherein the tenth amino acid is replaced by Met with Trp, and select the 4th to 21st amino acid sequence Th (4 to 21) of Thanatin. Lfcin B (1-15) is connected with Th (4-21) to design the antimicrobial peptide LF-TH, and then design the coding gene of the antimicrobial peptide LF-TH according to the codon preference of Escherichia coli;
[0018] 2. Link the gene encoding the antimicrobial peptide LF-TH to the plasmid pET-32a(+), construct a recombinant Escherichia coli expression vector, transform the host cell E.coli BL21(DE3), induce expression with IPTG, collect the cells by centrifugation, and ultrasonicate Cleavage and centrifuge to collect the supernatant, use Ni-NTA chromatography column and protein puri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com