A method and application of high-efficiency expression of recombinant hepatitis C virus multi-epitope antigen
A high-efficiency expression technology of hepatitis C virus, applied in the field of bioengineering, can solve problems such as high background value, poor uniformity, and misjudgment of results, and achieve the effect of increasing production and avoiding negative effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
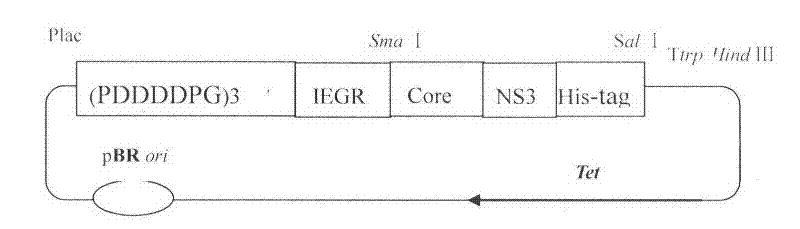
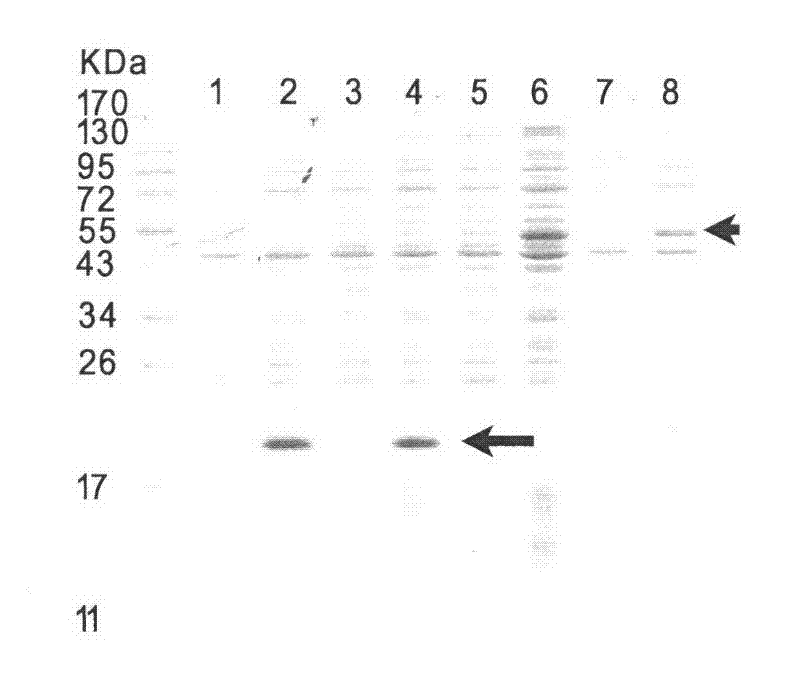
[0030] By conventional means of molecular biology, construct the expression vector containing Core protein, and contain Core-NS3 (such as figure 1 Shown) the expression vector of protein, two target proteins all use pET21b plasmid, Bl21 (DE3) Rosctta competent cell as expression strain. Specific operation: Transform pET21b-Core and pET21b-Core-NS3 plasmids into Bl21(DE3) Rosctta competent cells (double resistance to ampicillin and chloramphenicol). When cultured in LB medium at 37 degrees and 200 rpm to OD600 of about 0.6, 1 mM IPTG was added for induction for 2 hours. figure 2 Lanes 2 and 4 are the results of Core overexpression, lanes 6 and 8 are the results of Core-NS3 overexpression. 1, 3, 5, 7 are the controls before induction of 2, 4, 6, and 8, respectively. It can be seen from the SDS-PAGE figure that after induction, the target protein is expressed in large quantities, and the expression product is uniform. The Core protein sequence is SEQ ID NO.3, and the NS3 prot...
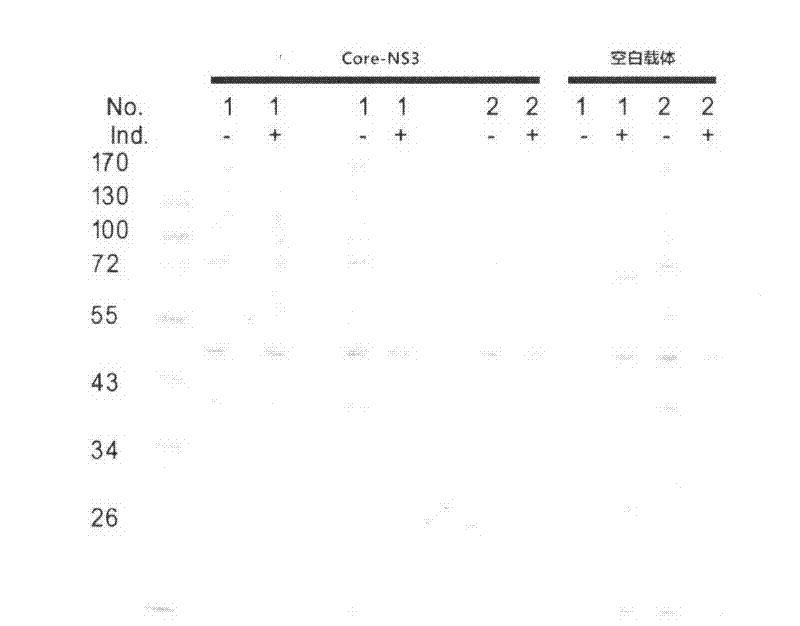
Embodiment 2
[0033]
[0034]
[0035]
[0036]
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com