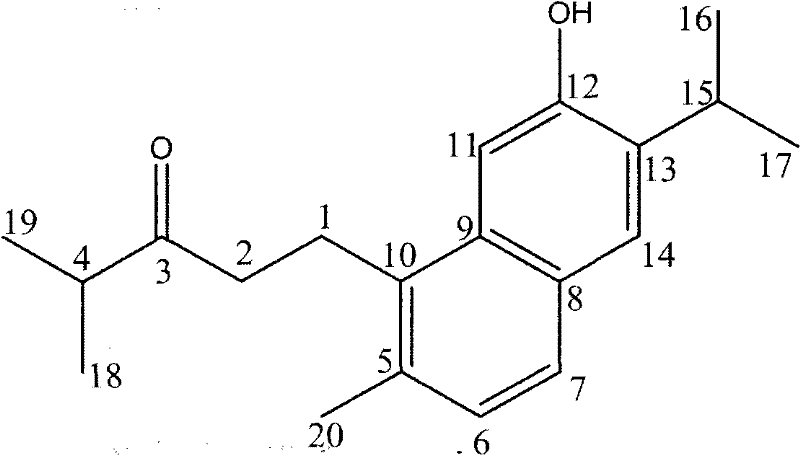
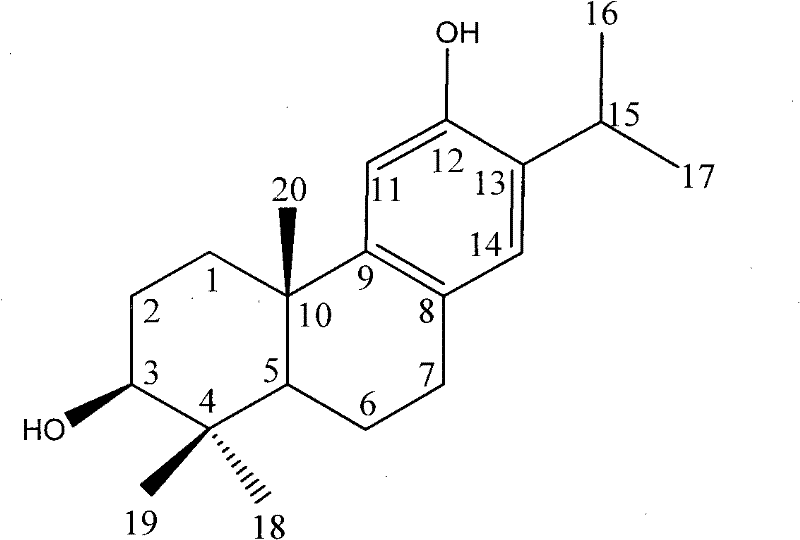
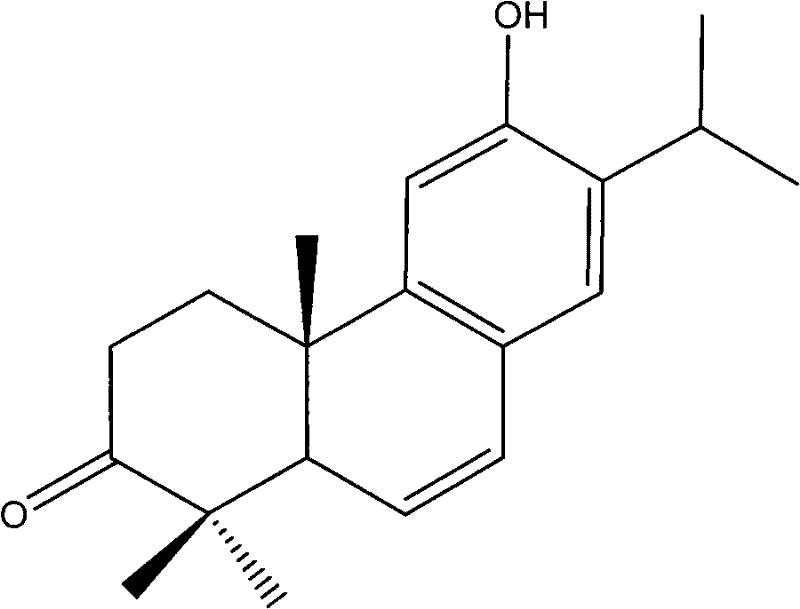
Method for biosynthesizing abietane diterpenoids by culturing cells from cloverleaf
A technique for rosinane diterpenes and culturing cells, which is applied in the field of inducing the biosynthesis of rosin alkane diterpenoids by liquid suspension culture cells of Tricuspid chinensis, can solve the problems of difficulty in obtaining, restricting research and application, low content and the like, and achieves improved purification. Efficiency, cost reduction, easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Cultivation of the cloverleaf cells:
[0039] Cells were cultured in suspension in liquid suspension:
[0040] The cell strain in the present invention is initially obtained from the suspension culture of the callus induced by the young stem of C. fortunei (C. fortunei), and the Murashige-Skoog (MS) medium is used, and the added concentration is 2 mg / L 2,4-dichloro Phenoxyacetic acid, 0.1mg / L kinetin and 30g / L sucrose, the pH value of the medium is 5.8. 7.5 grams of wet cells obtained by filtration were inoculated into a 500 ml shake flask containing 100 ml of the above medium, and then cultured on a rotary shaker at 100 rpm at 25° C. in the dark.
[0041] (2) Combined induced synthesis of abietane diterpenes:
[0042] On the 7th day of cell growth, methyl jasmonate (MJA) (final concentration in the medium was 300 μM / L) and XAD-7HP resin (100 g / L) were added to the medium, and the culture was continued until the 14th day, respectively. The cells and XAD-7HP were ...
Embodiment 2
[0097] The culture conditions and separation and purification conditions are the same as in Example 1, except that the difference from Example 1 is that MJA (final concentration in the medium is 150 μM / L) and XAD-7HP (100 g / L), continue to cultivate to 14 days, the results are as follows: compound 1 (5.6mg), compound 2 (15.5mg), compound 3 (17.4mg), compound 4 (5.5mg), compound 5 (220mg), compound 6 ( 5mg), Compound 7 (13mg)
Embodiment 3
[0099] The culture conditions and separation and purification conditions are the same as in Example 1, except that the difference from Example 1 is that jasmonic acid (final concentration in the medium is 150 μM / L) and XAD-7HP (100 g / L), continue to cultivate to 14 days, the results are as follows: compound 1 (7.5mg), compound 2 (16mg), compound 3 (20mg), compound 4 (3mg), compound 5 (190mg), compound 6 (6mg), Compound 7 (11.7 mg)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com