Medicinal composition for directional controlled release of trace elements and preparation method and application
A technology of trace elements and compositions, applied in the direction of drug combinations, pharmaceutical formulations, active ingredients of fluorine compounds, etc., can solve the problems of non-specific disease site-specific concentration demand contradictions, constraints, lack of efficiency in element intake and utilization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1 confirms that copper ions stimulate cell regeneration in vitro and stem cell mobilization experiments and the physiological effects of local film-controlled release of copper ions in the body
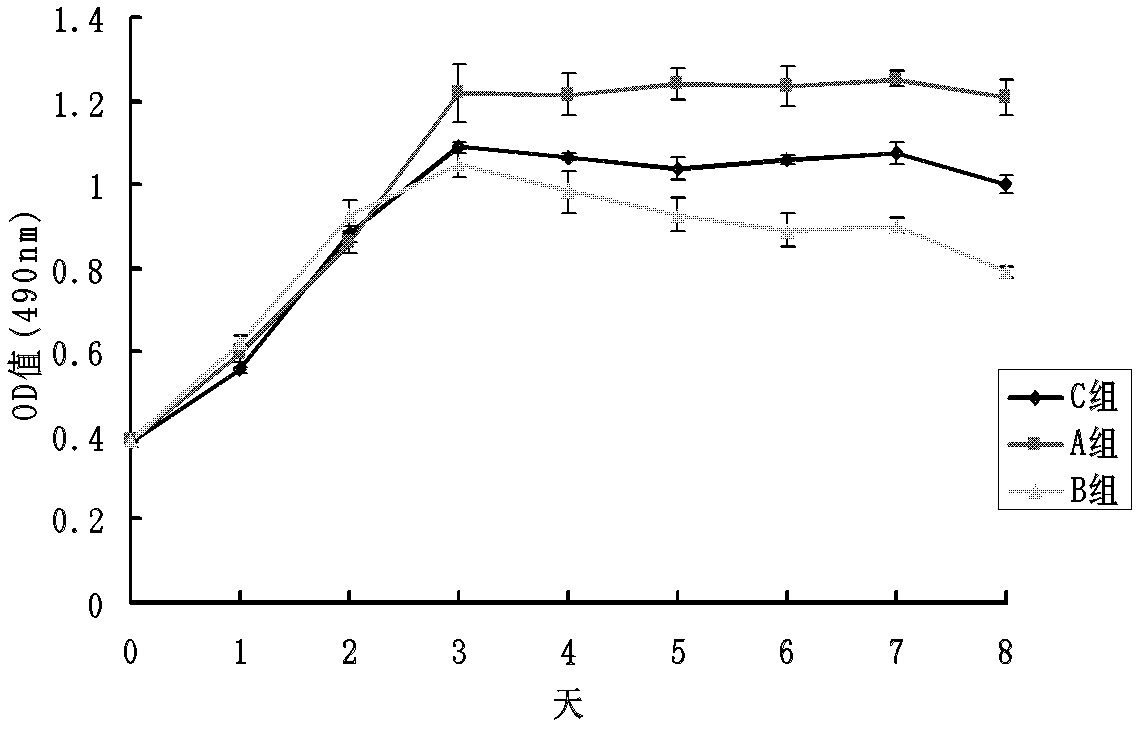
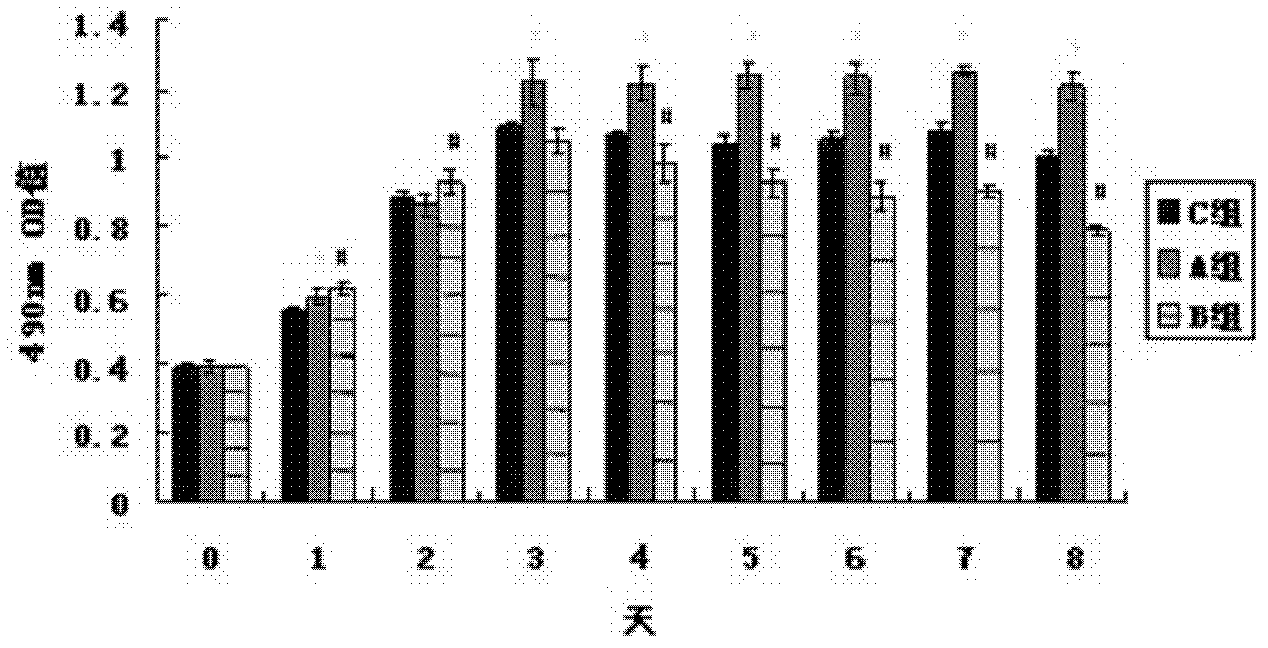
[0090] In vitro cell experiments have confirmed that trace element copper ions can effectively enhance the proliferation and differentiation of human umbilical vein endothelial cells (HUVEC). HUVECs were cultured and passaged in vitro. HUVEC in 5×10 3 Cells / well density were seeded in 96-well plates, and the cells were randomly divided into 3 groups according to the concentration of solutions added to the well plates, and the cells in group A were 5 μmol / L CuSO 4 , group B 25μmol / L CuSO 4 , group C is a blank control, each group has 4 duplicate wells, the basal medium is MCDB131, the cell proliferation is detected by MTT method and the growth curve is drawn. Take another HUVEC with 2×10 5 The cells / well density were seeded in 6-well plates, and the grouping was the ...
Embodiment 2
[0094] Example 2 confirms that copper ions stimulate cell regeneration and stem cell mobilization experiments in vitro and the physiological effects of local membrane-applied controlled release of copper ions in the body.
[0095] In order to determine the local effect of copper ions on the activation of scars and stimulate the regeneration of blood vessels in scars, the animal model of local myocardial infarction was pasted with copper ion gel to verify the physiological effect of local slow release of copper ions.
[0096] 1. Preparation of calcium alginate + CuSO 4 film. 10 ml of a 2% mass fraction of sodium alginate solution containing 10 mg of CuSO 4 The solution was mixed well, and 5ml CaCl was added dropwise 2 film after solution.
[0097] 2. Preparation of the myocardial infarction model: under anesthesia, the left anterior descending coronary artery of the rabbit heart was opened and ligated to prepare the rabbit left myocardial infarction model. In the state of t...
Embodiment 3
[0101] Example 3 Copper ion and albumin binding experiment (preparation of copper ion chelated albumin microbubbles)
[0102] The solution used in the experiment was prepared with deionized water, using 0.1mol L -1 NaCl maintains the ionic strength, uses Tris-HCl as the buffer solution, the pH value is 7.4, prepares HSA and copper sulfate solutions, the concentration is 8.76×10 -6 mol L·L-1 and 6.48×10 -4 mol L-1. Using a 1cm cuvette, measure (1) fix the excitation wavelength at 280nm, titrate HSA with copper sulfate, and observe the change of the fluorescence emission peak of HSA. It can be seen from the figure that Cu 2+ The addition of the copper sulfate did not significantly change the position of the excitation peak and the maximum fluorescence peak, but the fluorescence intensity weakened to varying degrees with the increase of the amount of copper sulfate. Since the volume of the HSA solution is much larger than the volume of the metal ion solution added dropwise, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com