Process for synthesizing 2-hydroxy-2-methyl-1-phenyl-1-propyl ketone
A synthesis process, the technology of acetone, applied in the preparation of carbon-based compounds, the preparation of organic compounds, organic chemistry, etc., can solve the problems of increased environmental pollution and low product yield, and achieve reasonable synthesis process and high product yield High and low environmental pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, a kind of synthetic technique of 2-hydroxyl-2-methyl-1-phenyl-1-propyl ketone, its steps are as follows:
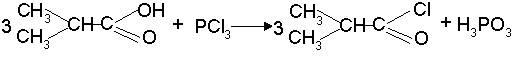
[0028] ⑴Acylation: Put isobutyric acid into the reaction kettle, raise the temperature to 45°C, add phosphorus trichloride, keep it at 48°C for at least 4 hours, remove the inorganic layer to obtain isobutyryl chloride; the raw material isobutyric acid and trichloride The weight ratio of phosphorus is 10:6.8;
[0029] (2) Friedel-Crafts reaction: Add benzene and catalyst aluminum trichloride to the reaction kettle, after cooling down to 5°C, add isobutyryl chloride dropwise, the weight ratio of benzene, aluminum trichloride and isobutyryl chloride is 9:5.0:4.0 , at 10°C for at least 2.5 hours to obtain a complex of 2-methyl-1-phenyl-1-propyl ketone and aluminum trichloride;
[0030] (3) Hydrolysis reaction: add the complex to an appropriate amount of dilute hydrochloric acid with a mass concentration of 1.5%, stir and separate layers, remove the aque...
Embodiment 2
[0034] Embodiment 2, a kind of synthetic technique of 2-hydroxyl-2-methyl-1-phenyl-1-propyl ketone, its steps are as follows:
[0035] ⑴Acylation: Put isobutyric acid into the reaction kettle, raise the temperature to 50°C, add phosphorus trichloride, keep it at 52°C for at least 4 hours, remove the inorganic layer to obtain isobutyryl chloride; the raw material isobutyric acid and trichloride The weight ratio of phosphorus is 10:7.2;
[0036] (2) Friedel-Crafts reaction: Add benzene and catalyst aluminum trichloride to the reaction kettle, after cooling down to 10°C, add isobutyryl chloride dropwise, the weight ratio of benzene, aluminum trichloride and isobutyryl chloride is 9:5.4:4.2 , at 15°C for at least 2.5 hours to obtain a complex of 2-methyl-1-phenyl-1-propyl ketone and aluminum trichloride;
[0037] (3) Hydrolysis reaction: Add the complex to an appropriate amount of dilute hydrochloric acid with a mass concentration of 2.5%, stir and separate layers, remove the aqu...
Embodiment 3
[0041] Embodiment 3, a kind of synthetic technique of 2-hydroxyl-2-methyl-1-phenyl-1-propyl ketone, its steps are as follows:
[0042] ⑴Acylation: Put isobutyric acid into the reaction kettle, raise the temperature to 47°C, add phosphorus trichloride, keep it at 50°C for at least 4 hours, remove the inorganic layer to obtain isobutyryl chloride; the raw material isobutyryl chloride and trichloride The weight ratio of phosphorus is 10:7;
[0043] (2) Friedel-Crafts reaction: Add benzene and catalyst aluminum trichloride to the reaction kettle, after cooling down to 8°C, add isobutyryl chloride dropwise, the weight ratio of benzene, aluminum trichloride and isobutyryl chloride is 9:5.2:4.1 , at 12°C for at least 2.5 hours to obtain a complex of 2-methyl-1-phenyl-1-propyl ketone and aluminum trichloride;
[0044] (3) Hydrolysis reaction: add the complex to an appropriate amount of dilute hydrochloric acid with a mass concentration of 2%, stir and separate layers, remove the aque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com