Preparation method and application method of naphthidine molecularly-imprinted polymer
A technology for imprinting polymers and binaphthylamine molecules is applied in separation methods, chemical instruments and methods, solid adsorbent liquid separation, etc. simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Preparation of Molecularly Imprinted Polymers
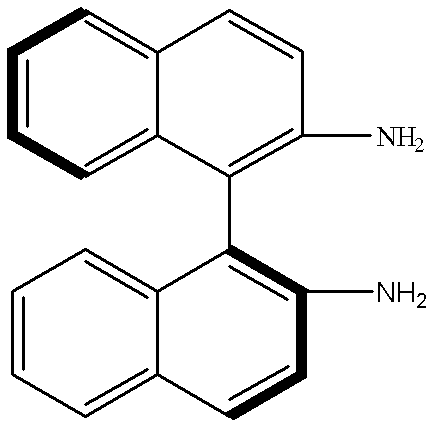
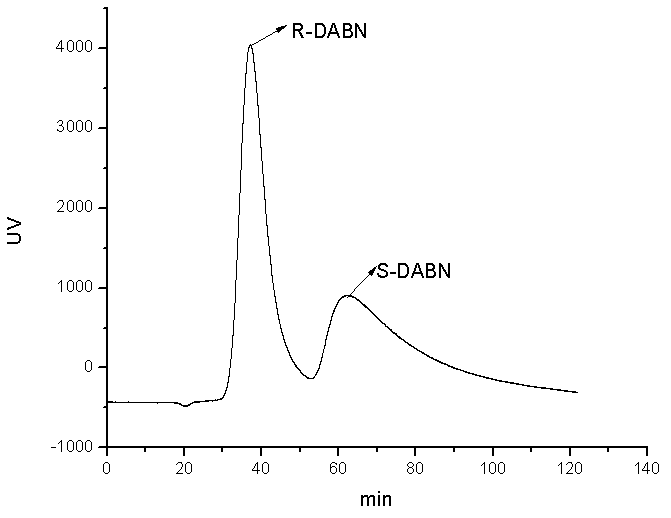
[0024] In a 100mL two-necked flask, add S-DABN (0.5mmol, 0.1422g) and MAA (2mmol, 0.17mL), then add acetonitrile: chloroform (1: 2, v / v) mixed solvent 6mL, make S-DABN and The MAA was completely dissolved, and nitrogen gas was passed for 5 minutes under ultrasonic conditions to remove oxygen and sealed. Pre-polymerize at 18°C for 10 hours under normal pressure to allow S-DABN and MAA to fully react to form a complex, add EDMA (10mmol, 1.89mL) and AIBN (20mg), and then pass nitrogen gas under ultrasonic conditions for 5 minutes to remove residual oxygen ,seal. Then place it in a constant temperature water bath at 60°C for 12 hours to initiate thermal polymerization to obtain a block molecularly imprinted polymer. Take out the block polymer, grind it, grind it, pass through a 60 μm-20 μm sieve, and settle it with acetone three times to remove very fine particles. , dried under vacuum at 60°C to obtain the S-DABN molec...
Embodiment 2
[0029] 1. Preparation of Molecularly Imprinted Polymers
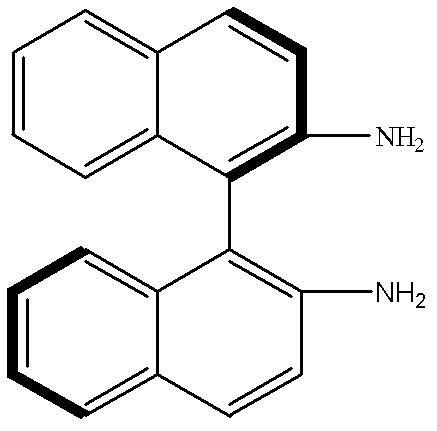
[0030] In a 100mL two-necked flask, add S-DABN (0.5mmol, 0.1422g) and MAA (2mmol, 0.17mL), then add acetonitrile: chloroform (1: 1, v / v) mixed solvent 6mL, make S-DABN and The MAA was completely dissolved, and nitrogen gas was passed for 5 minutes under ultrasonic conditions to remove oxygen and sealed. Pre-polymerize at 18°C for 10 hours under normal pressure to allow S-DABN and MAA to fully react to form a complex, add EDMA (10mmol, 1.89mL) and AIBN (20mg), and then pass nitrogen gas under ultrasonic conditions for 5 minutes to remove residual oxygen ,seal. Then place it in a constant temperature water bath at 60°C for 12 hours to initiate thermal polymerization to obtain a block molecularly imprinted polymer. Take out the block polymer, grind it, grind it, pass through a 60 μm-20 μm sieve, and settle it with acetone three times to remove very fine particles. , dried under vacuum at 60°C to obtain the S-DABN molec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com