Serum-free medium for culturing hepatic cells
A serum-free culture medium and culture medium technology, applied in animal cells, vertebrate cells, artificial cell constructs, etc., can solve the problems of unclear composition, unstable serum source, contamination of foreign viruses and pathogenic factors, etc. achieve good cell growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
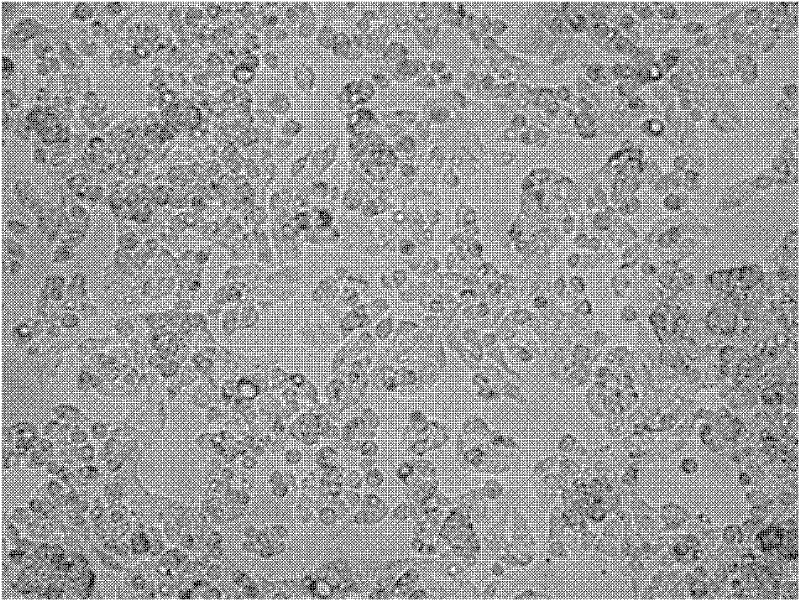
[0041] In this example, three groups of culture media were prepared, namely: serum-free culture medium, positive control group culture medium and negative control group culture medium. Among them, the medium of the positive control group was DMEM high glucose medium with 10% fetal bovine serum; the medium of the negative control group was DMEM high glucose medium. The cultured cells were human C3A cells. The following are the detailed experiments and detection steps:
[0042] 1. The configuration of the culture medium:
[0043] Serum-free medium: 5 mmol of HEPES, 10,000 U of penicillin and 10,000 U of streptomycin were added to each 500 mL of DMEM / F12 medium (provided by GIBCO, with a catalog number of 11330). Add supplemental components such that the concentration of supplemental components in the serum-free medium is:
[0044]
[0045]Positive control medium: 55 mL of fetal bovine serum, 5 mmol of HEPES, 10,000 U of penicillin and 10,000 U of streptomycin were added to...
Embodiment 2
[0064] The positive control medium, the negative control medium, the cell culture treatment method and the experimental result analysis method of Example 2 are the same as those in Example 1, and the difference is only that the added components in the serum-free medium are in the serum-free medium. The concentrations in the medium were replaced by:
[0065]
[0066]
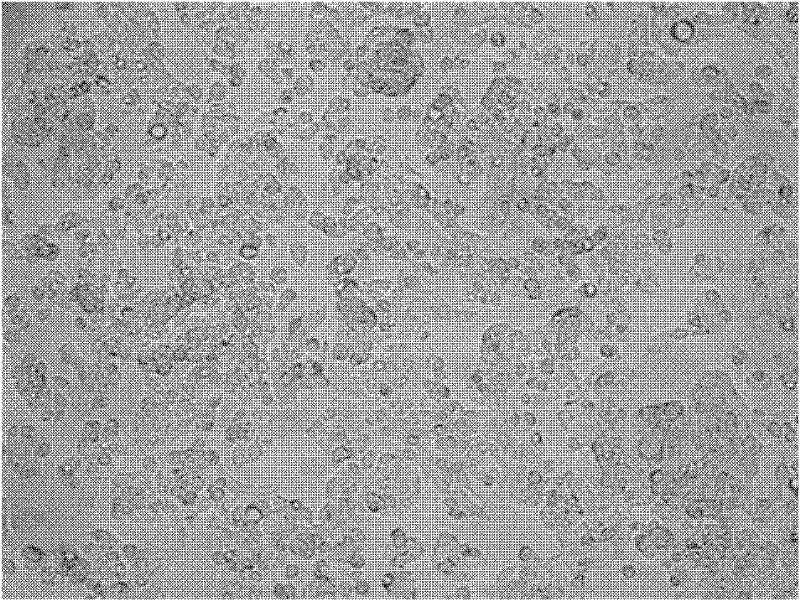
[0067] see Figure 7 , which are the growth curves of the C3A cells in the experimental group, the positive control group and the negative control group in Example 2. Among them, the C3A cells in the experimental group grew well, and the density of C3A cells in the positive control group was not significantly different, both of which were higher than those in the negative control group. Thus, it is indicated that the serum-free medium in the experimental group can effectively replace the role of serum and promote the proliferation of cells.
[0068] see Figure 8 , which is a graph of cell viability meas...
Embodiment 3
[0073] The positive control medium, the negative control medium, the cell culture treatment method and the experimental result analysis method of Example 3 are the same as those in Example 1, and the difference is only that the added components in the serum-free medium are in the serum-free medium. The concentrations in the medium were replaced by:
[0074]
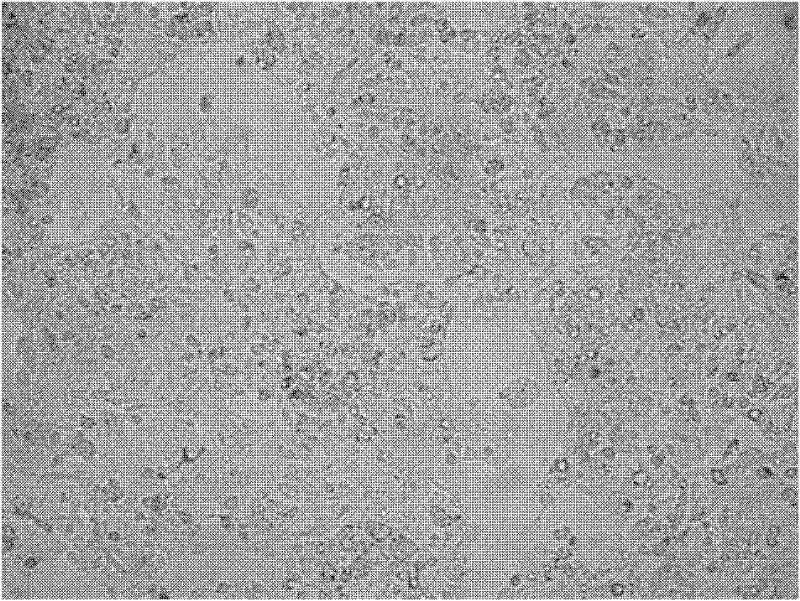
[0075] see Figure 12, which are the growth curves of C3A cells in the experimental group, the positive control group and the negative control group in Example 3. Among them, the C3A cells in the experimental group grew well, and the density of C3A cells in the positive control group was not significantly different, both of which were higher than those in the negative control group. Thus, it is indicated that the serum-free medium in the experimental group can effectively replace the role of serum and promote the proliferation of cells.
[0076] see Figure 13 , which is a graph of cell viability measured by MTT met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com