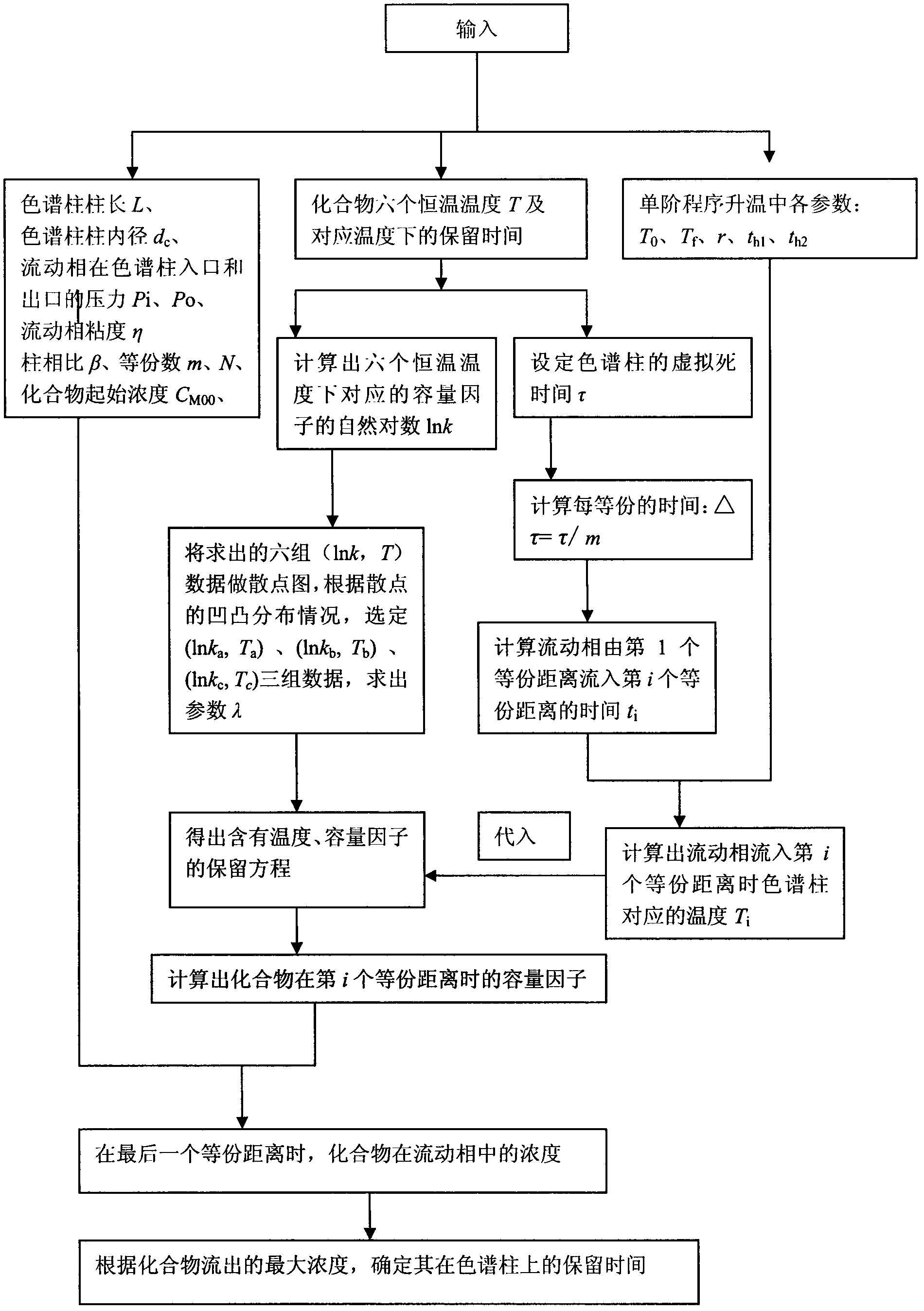
Method for predicting retention time of gas phase chromatogram based on macromolecule crystallization behavior derivation retention equation
A technology of retention time and gas chromatography, which is applied in the field of gas chromatography, can solve problems such as few data points, obvious deviation of retention equation, large retention time prediction error, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Instruments: HP6890 gas chromatograph, hydrogen flame ionization detector, 6890 gas chromatograph workstation;
[0113] Chromatographic column: non-polar HP-5 chromatographic column (5% phenylmethyl polysiloxane, 30m × 0.32mm × 0.25μm, Agilent Technologies Co., Ltd.);
[0114] Conditions: The temperature of the detector is 250°C, and the temperature of the injection port is 250°C;
[0115] Carrier gas: use high-purity nitrogen (purity not less than 99.999%);
[0116] Injection method: split injection, the split ratio is 50:1, each injection volume is 0.2ul, and the concentration is 1μg / ml;
[0117] Constant flow operation mode: the carrier gas is at the outlet of the column, and the mass flow rate is kept constant at 1.0ml / min;
[0118] Choose from three different temperature programs, which are:
[0119] A Program temperature rise 30℃→25℃ / min→250℃
[0120] B Program temperature rise 30℃→15℃ / min→250℃
[0121] C program temperature rise 30℃→5℃ / min→250℃
[0122] (1)...
Embodiment 2
[0138] The process and condition of the present embodiment are identical with embodiment 1, and difference is:
[0139] (1) The virtual dead time τ is 1.40min;
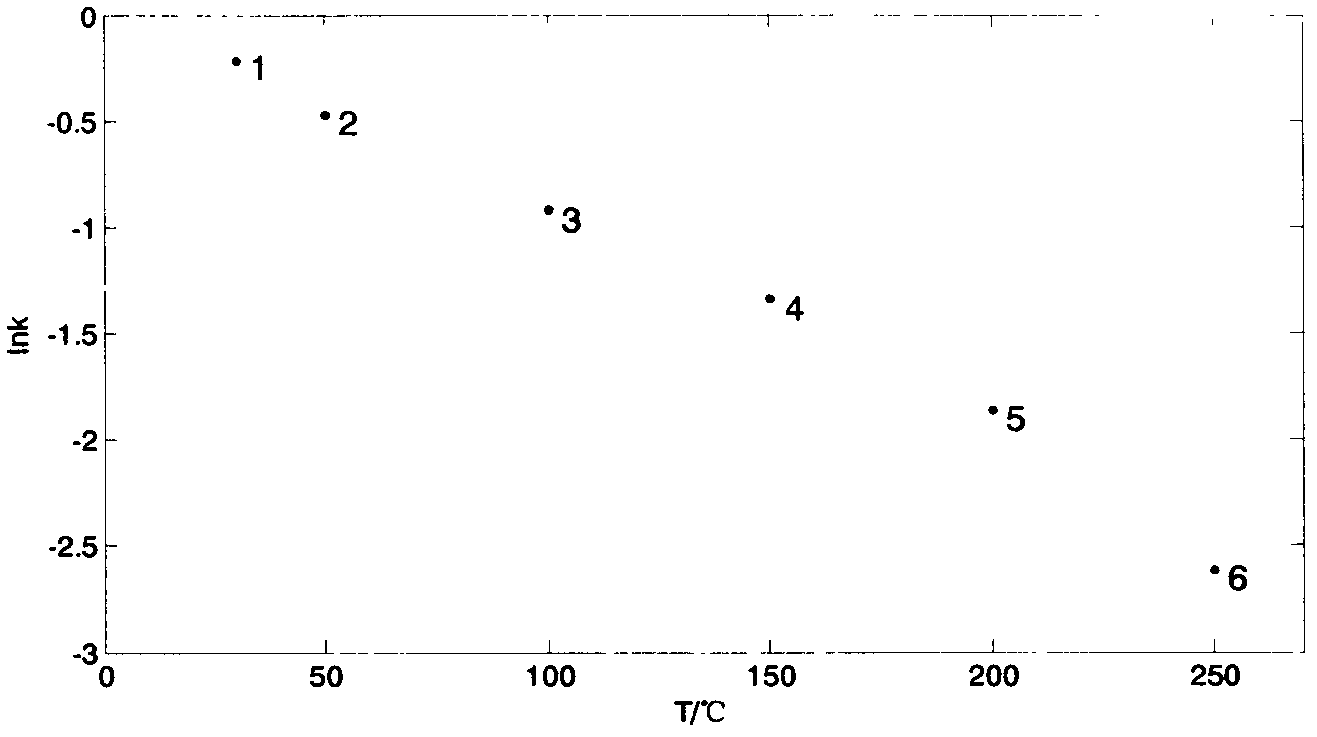
[0140](2) Calculate the capacity factors of methanol, heptane, and isoamyl acetate at six constant temperatures of 250°C, 200°C, 150°C, 100°C, 50°C, and 30°C according to formula 13 and take the natural logarithm. The natural logarithms of the capacity factors of methanol at corresponding temperature points are: -0.868, -0.637, -0.401, -0.157, 0.141, 0.329; the natural logarithms of the capacity factors of heptane at corresponding temperature points are: -0.829, -0.573 .
[0141] (3) Make scatter diagrams of the lnk of methanol, heptane, and isoamyl acetate changing with temperature T, respectively. Three data points selected for methanol (lnk a ,T a ), (lnk b ,T b ) and (lnk c ,T c ) are (0.329, 30°C), (-0.157, 100°C) and (-0.401, 150°C); the three data points selected for heptane are (1.613, 30°C), (0.133, 1...
Embodiment 3
[0151] The process and condition of the present embodiment are identical with embodiment 1, and difference is:
[0152] (1) The virtual dead time τ is 0.90min;
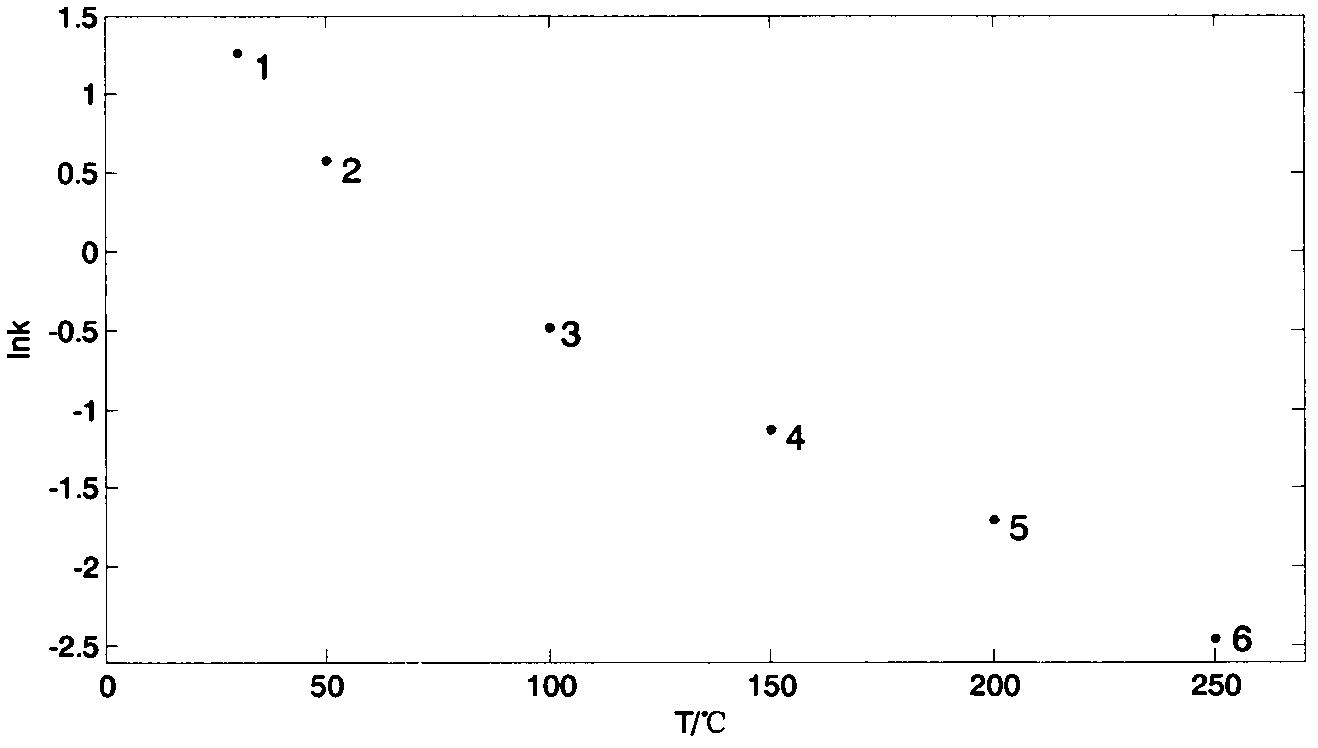
[0153] (2) Calculate the capacity factors of methanol, heptane, and isoamyl acetate at six constant temperatures of 250°C, 200°C, 150°C, 100°C, 50°C and 30°C according to formula 13 and take the natural logarithm. The natural logarithms of capacity factors at corresponding temperature points are: 0.189, 0.321, 0.468, 0.634, 0.853, 1.000; ; The natural logarithms of capacity factors of isoamyl acetate at corresponding temperature points are: 0.233, 0.398, 0.649, 1.205, 2.639, 3.617.
[0154] (3) Make scatter diagrams of the lnk of methanol, heptane, and isoamyl acetate changing with temperature T, respectively. Three data points selected for methanol (lnk a ,T a ), (lnk b ,T b ) and (lnk c ,T c ) are: (1.000, 30°C), (0.634, 100°C) and (0.468, 150°C); the three data points selected for heptane are (2.124, 30°C),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com