Chinese medicinal composition for treating chronic hepatitis B and preparation method and application thereof
A technology for chronic hepatitis B and the composition is applied in the field of traditional Chinese medicine compositions for the treatment of chronic hepatitis B and the field of preparation thereof, which can solve the problems of no curative effect and the like, and achieve the effects of improving clinical symptoms, definite curative effect and accurate compatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
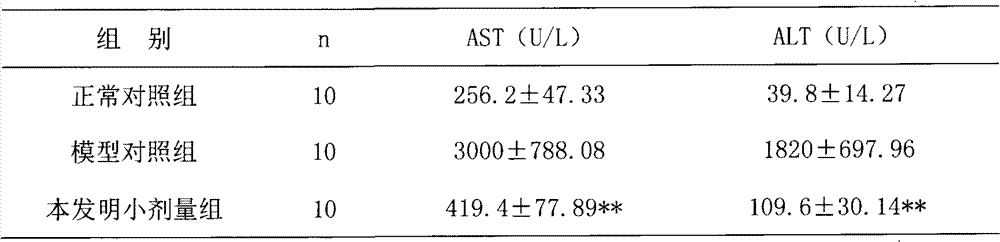
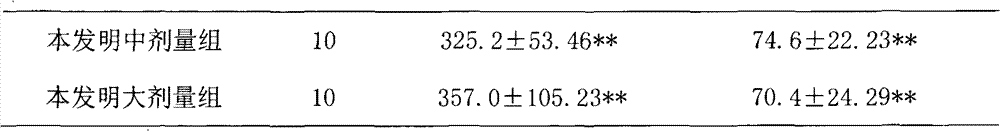
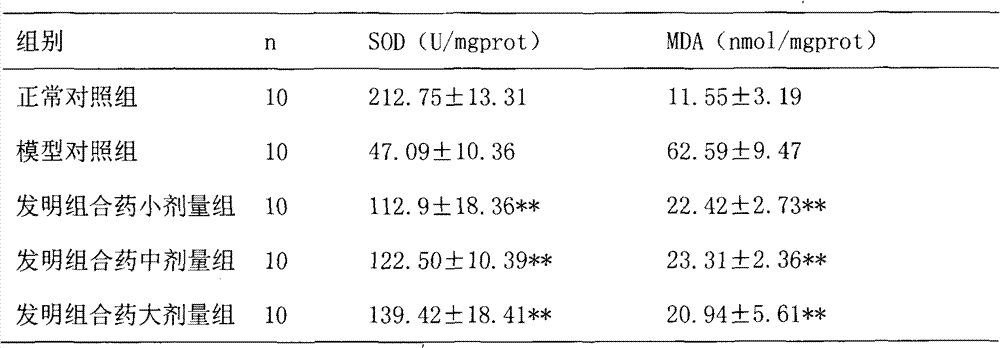
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of capsule
[0031] According to the raw material ratio in Table 1 and the conventional method of capsule dosage form, it is prepared into capsules, and the specific methods are as follows:
[0032] Take 10g of Solanum nigrum, 10g of Bupleurum radix, 20g of Hedyotis diffusa, 40g of Hedyotis diffusa, 10g of Ligustrum lucidum, 11g of jasmine jasmine, and 3g of licorice. 8 times the amount, decoct for 1 hour, add water for the second time to 6 times the weight of the medicinal materials, decoct for 1, combine the decoction, concentrate to 65 ° C, the relative density is 1.15, add ethanol to make the volume percentage of the alcohol content reach 75% , stirred, left to stand, filtered, the relative density of the filtrate was 1.20 when the filtrate was concentrated under reduced pressure to 65°C, and ethanol was recovered to obtain a concentrated solution, which was spray-dried to obtain a dry extract powder. The spray-drying conditions were: t...
Embodiment 2
[0033] Embodiment 2: the preparation of granule
[0034]According to the ratio of raw materials in Table 1 and the conventional method of granule dosage form, it is prepared into granules, and the specific method is as follows:
[0035] Take 15g Solanum nigrum, 8g Bupleurum bupleurum, 30g Hedyotis diffusa, 30g Weeping grass, 12g Ligustrum lucidum, 9g Jasmine jasmine, and 5g licorice. 10 times the amount, decocting for 1.5 hours, adding water for the second time is 8 times the weight of the medicinal materials, decocting for 1.5 hours, combining the decoctions, concentrating to 65 °C, the relative density is 1.20, adding ethanol to make the volume percentage of the alcohol content reach 80%, stirring, standing still, filtering, the relative density of the filtrate is 1.25 when the filtrate is concentrated under reduced pressure to 65°C and the ethanol is recovered to obtain a concentrated solution, which is spray-dried to obtain a dry extract powder. The spray-drying conditions...
Embodiment 3
[0036] Embodiment 3: the preparation of tablet
[0037] Prepare by the raw material proportioning of table 1 and tablet dosage form conventional method, be prepared into tablet, concrete method is as follows:
[0038] Take 20g of Solanum nigrum, 6g of Bupleurum radix, 40g of Hedyotis diffusa, 20g of Hedyotis diffusa, 14g of Ligustrum lucidum, 7g of Jasmine jasmine, and 7g of licorice. 12 times the amount, decoct for 2 hours, add water for the second time to 10 times the weight of the medicinal materials, decoct for 2 hours, combine the decoction, concentrate to 65 ° C, the relative density is 1.20, add ethanol to make the volume percentage of the alcohol content reach 85% , stir, stand still, filter, the relative density of the filtrate is 1.30 when the filtrate is concentrated under reduced pressure to 65°C and the ethanol is recovered to obtain a concentrated solution, which is prepared into an oral liquid by adding water and auxiliary materials to the concentrated solution,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com