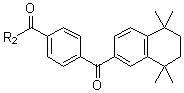
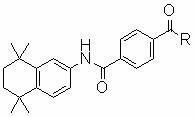
Multiple-target-point tamibarotene derivatives, preparation method and purposes thereof
A tamibarotene, multi-target technology, applied in the multi-target tamibarotene derivatives and their preparation, in the field of anti-malignant tumor (especially leukemia) drugs, can solve the problem of skin irritation and labor Protection conditions require high, low drug resistance and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
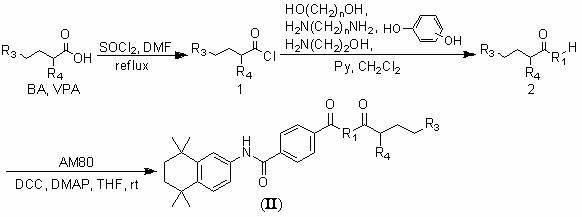
[0063] Example 1: Butyryl chloride ( 1a ) and 2-propylpentanoyl chloride ( 1b ) preparation
[0064] Add n-butyric acid (88.1g, 1.0mol) or valproic acid (144.2g, 1.0mol) to thionyl chloride (119.0g, 1.0mol), then add 0.1mL DMF as a catalyst, and stir the reaction at 60°C for 1 Hours, distill and collect n-butyryl chloride (collect 95~105°C fraction by atmospheric distillation) or 2-propylpentanoyl chloride (collect 75~85°C / 2.6kPa fraction by vacuum distillation) to obtain a colorless transparent liquid ( 1a ) (n-butyryl chloride, 99.7g, yield 93.6%) and ( 1b ) (2-propylpentanoyl chloride, 147.8g, yield 90.9%), which was directly used in the next reaction.
[0065]
Embodiment 2
[0066] Example 2: 2-Hydroxyethylbutyrate ( 2a ) and 2-hydroxyethyl-2-propylvalerate ( 2d ) preparation
[0067] Butyryl chloride ( 1a ) (10.7g, 0.1mol) or 2-propylpentanoyl chloride ( 1b ) (16.3g, 0.1mol) was dissolved in 50 mL of dichloromethane to obtain solution 1, and ethylene glycol (0.1mol) and triethylamine (15.2g, 0.15mol) were added to 100mL of dichloromethane to obtain solution 2. Slowly add solution 1 to solution 2 with stirring at room temperature, and the dropwise addition is completed within 2 hours, then stir at room temperature for 5 hours, concentrate under reduced pressure and distill off the solvent and excess triethylamine, the residue is separated by column chromatography, and the eluent is reduced to Concentrate under pressure to dryness to obtain pure product 2-hydroxyethyl butyrate ( 2a ) (yield 48.1%) and 2-hydroxyethyl-2-propylvalerate ( 2d ) (yield 54.8%), directly used in the next reaction.
[0068]
Embodiment 3
[0069] Example 3: N-(2-aminoethyl)butyramide ( 2g ) and N-(2-aminoethyl)-2-propylpentanamide ( 2h ) preparation
[0070] Ethylenediamine (0.1mol) and triethylamine (15.2g, 0.15mol) were added to 100mL of dichloromethane to obtain solution 1, n-butyryl chloride ( 1a ) (10.7g, 0.1mol) or 2-propylpentanoyl chloride ( 1b ) (16.3g, 0.1mol) was dissolved in 50 mL of dichloromethane to obtain solution 2. Slowly add solution 2 to solution 1 under stirring at room temperature, the dropwise addition is completed within 2 hours, then stir at room temperature for 3 hours, concentrate under reduced pressure to remove the solvent and excess triethylamine, the residue is separated by column chromatography, and the eluent is reduced to Concentrate under reduced pressure to dryness to obtain pure product N-(2-aminoethyl) butyramide ( 2g ) (yield 56.7%) and N-(2-aminoethyl)-2-propylpentanamide ( 2h ) (yield 63.5%), directly used in the next reaction.
[0071]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com