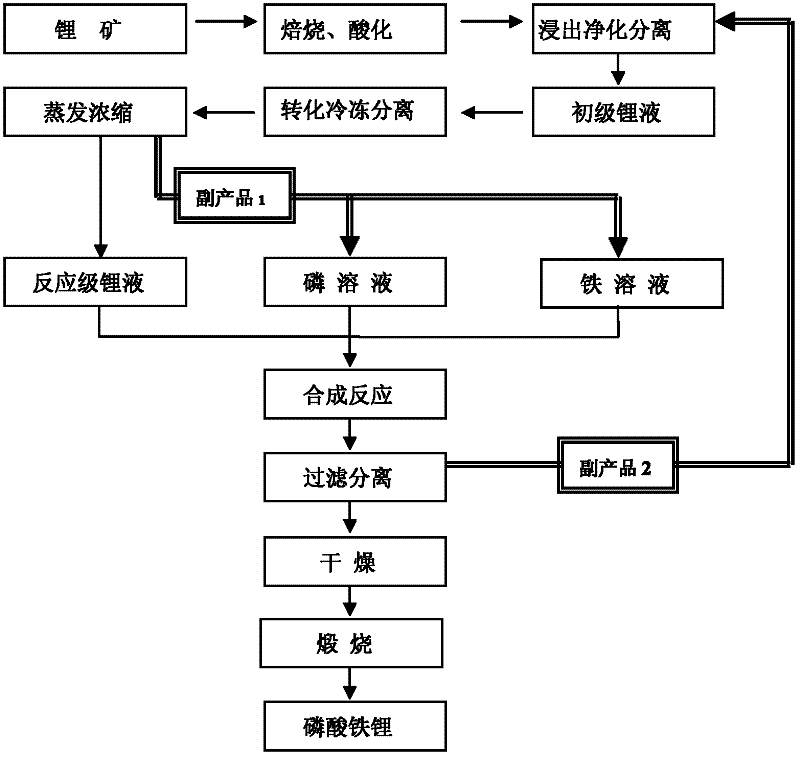
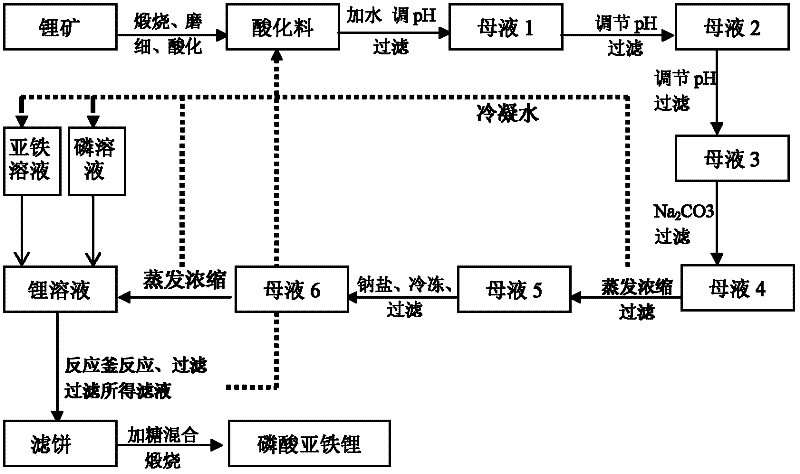
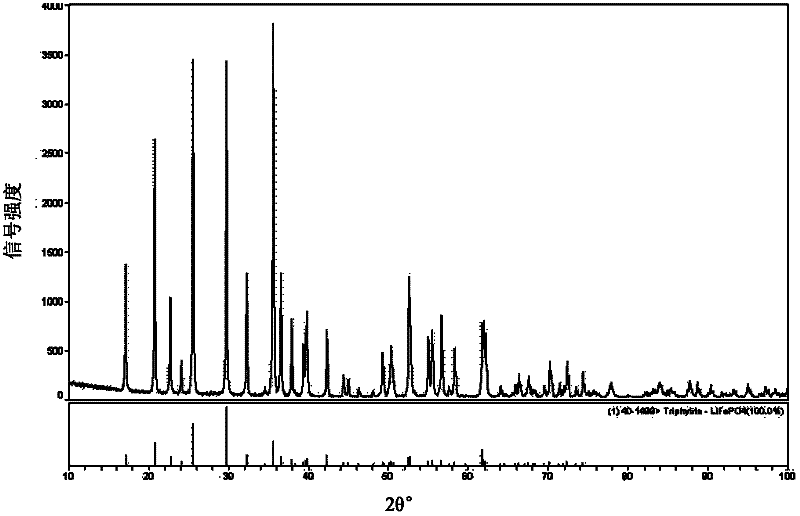
Method for producing lithium ferrous phosphate by using lithium mine as lithium source
A technology of lithium iron phosphate and lithium ore, which is applied in the field of preparation of lithium iron phosphate, can solve the problems of high cost of lithium iron phosphate, and achieve the effects of realizing circular economy, reducing production costs, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 Preparation of lithium iron phosphate
[0098] (1) Weigh 50kg of spodumene, calcinate at 1100°C for 50 minutes, cool, grind, add 7.1kg of sulfuric acid (acid to material ratio 1:7) for 50 minutes, pour 114kg (liquid-solid ratio 2 : 1) In water, adjust the pH value to 5.7 with NaOH, stir for 35 minutes, let stand, and filter to obtain mother liquor 1;
[0099] (2) Adjust the pH value of mother liquor 1 to 8.5 with NaOH, stir and react for 5 minutes, let stand, filter to obtain mother liquor 2, then adjust the pH value of mother liquor 2 to 10.8 with NaOH, stir and react for 5 minutes, stand still, filter to obtain mother liquor 3; add 236.6 grams of Na 2 CO 3 , stirring and reacting for 30 minutes, standing still, and filtering to obtain mother liquor 4;
[0100] (3) Evaporate and concentrate the mother liquor 4 until its lithium content is 65g / L, leave it still, filter to obtain the mother liquor 5, wherein the condensed water obtained by evaporation and ...
Embodiment 2
[0112] The preparation of embodiment 2 lithium ferrous phosphate
[0113] (1) Weigh 50kg of lithium phosphate aluminum, calcined at 1380°C for 300 minutes, cooled, ground, added 12.5kg of sulfuric acid (according to the ratio of acid to material 1:4) for 200 minutes, poured into 187.5kg of recovery filtrate while stirring Medium (liquid-solid ratio 3:1), adjust the pH value to 6.2 with NaOH, stir for 50 minutes, let stand, and filter to obtain mother liquor 1;
[0114] (2) Adjust the pH value of mother liquor 1 to 9.7 with NaOH, stir and react for 12 minutes, let stand, filter to obtain mother liquor 2, then adjust the pH value of mother liquor 2 to 10 with NaOH, stir and react for 12 minutes, stand still, filter to obtain mother liquor 3; add 240.5 grams of Na 2 CO 3 , stirred and reacted for 10 minutes, allowed to stand, and filtered to obtain mother liquor 4;
[0115] (3) Evaporate and concentrate the mother liquor 4 until its lithium content is 75g / L, leave it still, fi...
Embodiment 3-9
[0124] The preparation of embodiment 3-9 lithium ferrous phosphate
[0125] The preparation process of Examples 3-9 is the same as that of Example 1, and the required preparation materials and process parameters are shown in Table 2.
[0126] Preparation raw materials and process parameters of table 2 embodiment 3-9
[0127]
[0128] As can be seen from Table 2, according to the assay method of the present invention, the purity of Lithium Ferrous Phosphate is not less than 99.97%, and Ca in Lithium Ferrous Phosphate 2+ , Mg 2+ , SO4 2- , Cl - 、Na + 、K + 、Cu 2+ , Pb 2+ The content of any one is not higher than 0.01%, and the 1C specific capacity is more than 140mAh / g. It can be seen that the lithium ferrous phosphate prepared by the process of the present invention has the advantages of high purity, excellent electrochemical performance, good stability and consistency.
[0129] Moreover, the process of the present invention can control the concentration of lithium io...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com