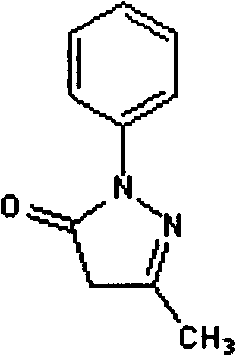
Edaravone compound with stable crystal form
A compound and crystal form technology, applied in the field of edaravone compounds, can solve the problems of insoluble edaravone and the like, and achieve the effects of simple and easy method, good stability and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Put 5.0 g of crude Edaravone into a single-mouth bottle, add 80 ml of pure ethanol aqueous solution (ethanol: pure water = 1:3), and stir to dissolve at 45°C;
[0033] 2) Then adjust the pH of the Edaravone solution to 4.0 with hydrochloric acid at room temperature, add 600ml of diethyl ether and acetone mixture (diethyl ether: acetone = 1:2.5), and stir and crystallize at 20°C for 3 hours;
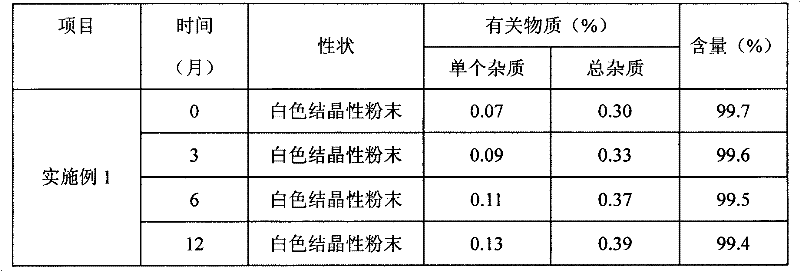
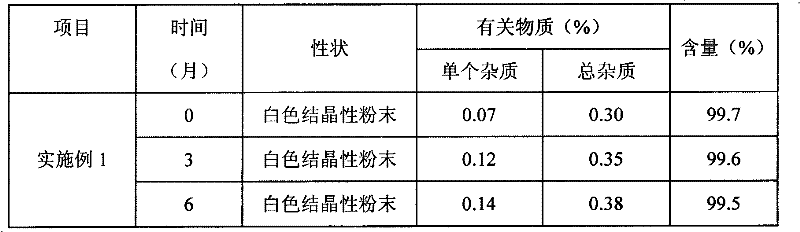
[0034] 3) The precipitated Edaravone crystals were collected by filtration, washed with 100ml of the above ether and acetone mixture and dried in an oven at 30°C to obtain 4.5g of Edaravone crystals with a yield of 90% and a purity of 99.7%.
Embodiment 2
[0036] 1) Put 5.0 g of crude Edaravone into a single-mouth bottle, add 100 ml of pure ethanol aqueous solution (ethanol: pure water = 1:3), and stir to dissolve at 48°C;
[0037] 2) Then adjust the pH of the Edaravone solution to 4.2 with hydrochloric acid at room temperature, add 500ml of isopropyl ether and acetone mixture (isopropyl ether: acetone = 1:3), stir and crystallize at 30°C for 5 hours;
[0038] 3) The precipitated Edaravone crystals were collected by filtration, washed with 100ml of the above isopropyl ether and acetone mixture and dried in an oven at 30°C to obtain 4.55g of Edaravone crystals with a yield of 91% and a purity of 99.6 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com