Preparation method of ion vanadium redox battery electrolyte
A liquid flow battery and vanadium ion technology, applied in the direction of indirect fuel cells, etc., can solve the problems of high cost, reduced electrolyte cost, complicated operation, etc., and achieve the effect of low cost, good electrochemical performance, and simple and easy-to-operate preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
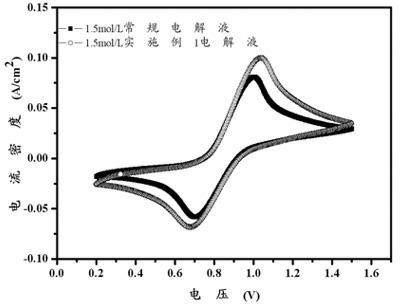
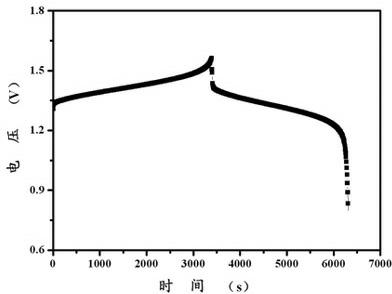
[0025] Dissolve 0.3 mol of ethanol with 500 ml of deionized water to obtain an aqueous solution of an organic reducing agent, then weigh 0.75 mol of vanadium pentoxide and 4 mol of sulfuric acid and mix to obtain a suspension. Add the aqueous solution of the organic reducing agent to the mixed suspension of vanadium pentoxide and sulfuric acid aqueous solution, seal the outlet of the reaction vessel, heat and stir, and control the temperature at 60°C; after the reaction, open the outlet of the vessel and continue heating for 30 minutes. The temperature range is controlled at 120°C; after the solution is cooled to room temperature, deionized water is added to make the volume to 1L, and a 1.5mol / L vanadium battery electrolyte is obtained. Graphite is used as the positive and negative electrode material, and the concentration of the positive and negative electrode solutions is 1.5mol / L. The vanadium battery electrolyte has a charging current density of 20mA / cm 2 , the discharge c...
Embodiment 2
[0027] Dissolve 0.2 mol of acetaldehyde with 500 ml of deionized water to obtain an aqueous solution of an organic reducing agent, then weigh 0.75 mol of vanadium pentoxide and 4 mol of sulfuric acid and mix to obtain a suspension. Add the aqueous solution of the organic reducing agent to the mixed suspension of vanadium pentoxide and sulfuric acid aqueous solution, seal the outlet of the reaction vessel, heat and stir, and control the temperature at 50°C; after the reaction, open the outlet of the vessel and continue heating for 30 minutes. The temperature range is controlled at 120°C; after the solution is cooled to room temperature, deionized water is added to make the volume to 1L, and a 1.5mol / L vanadium battery electrolyte is obtained. Graphite is used as the positive and negative electrode material, and the concentration of the positive and negative electrode solutions is 1.5mol / L. The vanadium battery electrolyte has a charging current density of 45mA / cm 2 , the discha...
Embodiment 3
[0029] Dissolve 0.15 mol of acetaldehyde and 0.15 mol of isopropanol with 500 ml of deionized water to obtain an aqueous solution of an organic reducing agent, then weigh 0.75 mol of vanadium pentoxide and 4 mol of sulfuric acid and mix to obtain a suspension. Add the aqueous solution of the organic reducing agent to the mixed suspension of vanadium pentoxide and sulfuric acid aqueous solution, seal the outlet of the reaction vessel, heat and stir, and control the temperature at 60°C; after the reaction, open the outlet of the vessel and continue heating for 60 minutes. The temperature range is controlled at 150°C; after the solution is cooled to room temperature, deionized water is added to make the volume to 1L to obtain a 1.5mol / L vanadium battery electrolyte. Graphite is used as the positive and negative electrode material, and the concentration of the positive and negative electrode solutions is 1.5mol / L. The vanadium battery electrolyte has a charging current density of 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com