Liposome composition
A liposome composition and liposome technology, applied in the direction of liposome delivery, drug combination, inorganic non-effective ingredients, etc., can solve the leakage of active compounds, the low stability of active compounds, and the difficulty of achieving high active compounds at the same time The encapsulation rate of active compounds maintains stability and other issues, and achieves the effect of maintaining high stability and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0137] Preparation of liposomes includes lipid membrane method (Vortex method), reverse phase evaporation method, ultrasonic method, Pre-vesicle method, ethanol injection method, French Press method, bile acid removal method, Triton X-100 batch method Law, Ca 2+ Fusion method, ether injection method, annealing method, freeze-thaw fusion method, etc.
[0138] Various conditions in the preparation of liposomes (amounts of membrane constituents, temperature, etc.) can be appropriately determined according to the preparation method of liposomes or the composition, particle size, etc. of target liposomes (see the aforementioned Kikuchi (1983) Wait).
[0139] The particle size of liposomes can be adjusted arbitrarily as needed. For example, the particle size can be adjusted by extruding under high pressure (extrusion filtration) using a membrane filter having a uniform pore size. The adjustment of the particle size can be carried out at any time during the manufacture of the lipo...
Embodiment 1
[0186]
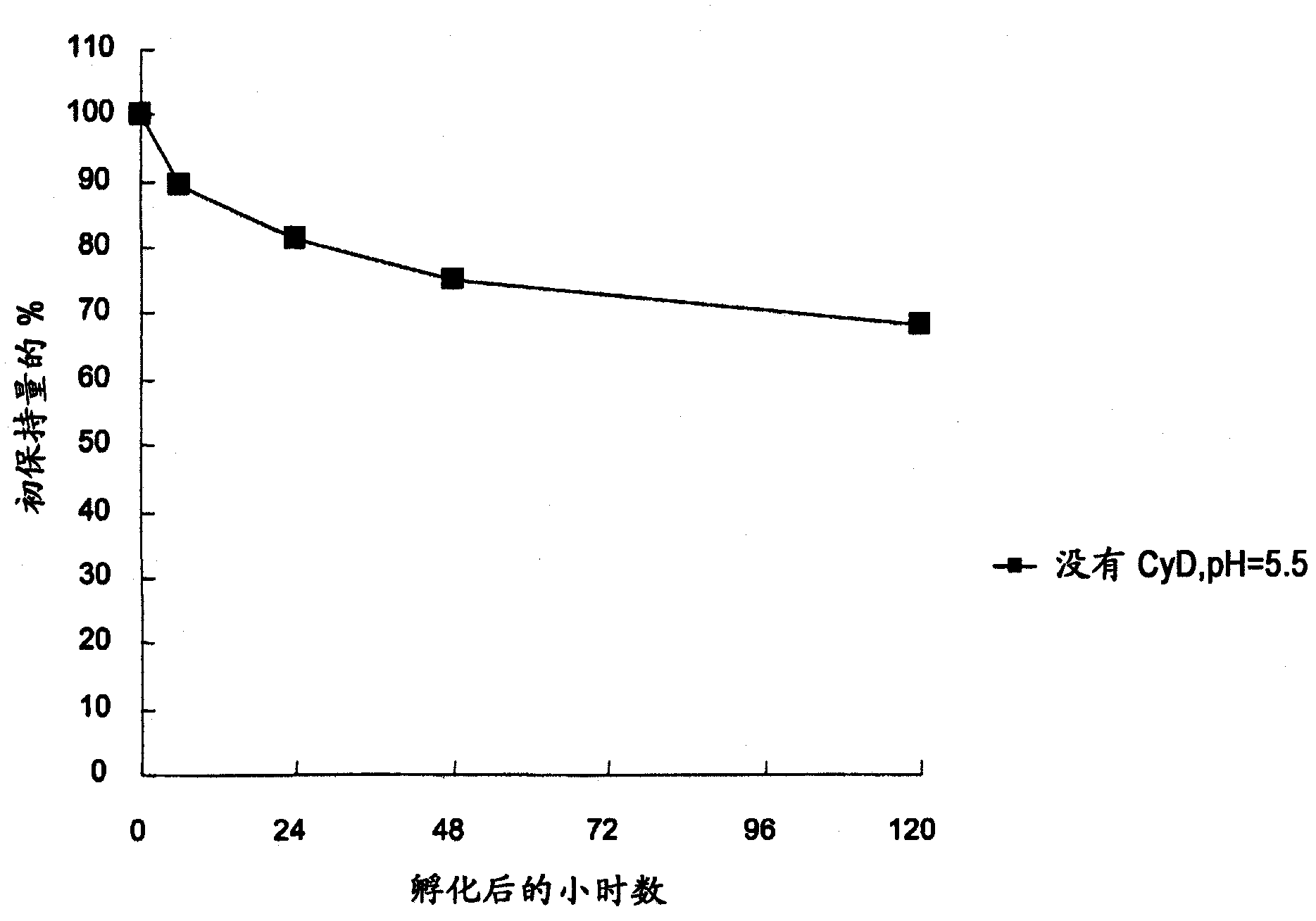
[0187] 396.4 mg of ammonium sulfate and 189.1 mg of citric acid monohydrate were dissolved in pure water and diluted to 15 mL to prepare a 200 mM ammonium sulfate / 60 mM citric acid aqueous solution. An aqueous solution for the liposome inner phase was prepared by adjusting the pH of 2.5 mL of a 200 mM ammonium sulfate / 60 mM citric acid aqueous solution to 5.5 with ammonia water and then diluting to 5 mL with pure water.
[0188]
[0189] 317.9 mg of hydrogenated soybean lecithin (manufactured by Lipoid), 116.0 mg of cholesterol (manufactured by Sigma), and 130.4 mg of polyethylene glycol 2000-phosphatidylethanolamine (manufactured by Genzyme, MPEG2000-distearoylphosphatidylethanolamine) were dissolved in 10 mL of chloroform, and then accurately divided into 3 parts, one of which was distilled off chloroform using a rotary evaporator under reduced pressure to form a lipid film. To the obtained lipid film, 5 mL of an aqueous solution for liposome inner phase heated ...
Embodiment 2
[0203]
[0204] In the same manner as in Example 1, 264.3 mg of ammonium sulfate and 126.1 mg of citric acid monohydrate were dissolved in pure water and diluted to 10 mL using a volumetric flask to prepare a 200 mM ammonium sulfate / 60 mM citric acid aqueous solution. 1 mL was measured therefrom, adjusted to pH 5.5 with aqueous ammonia, and then diluted to 2 mL with pure water to prepare an aqueous solution for liposome internal phase.
[0205]
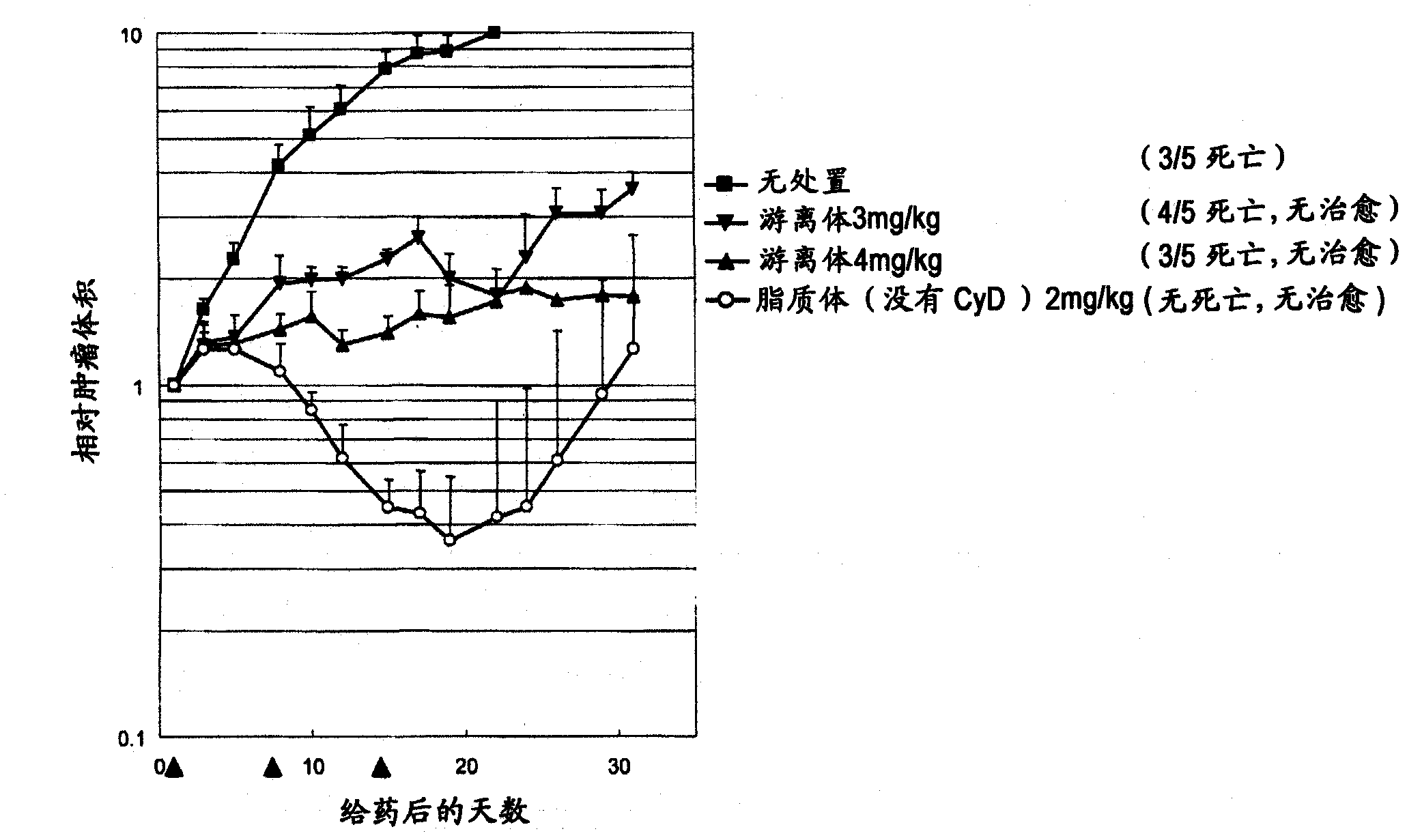
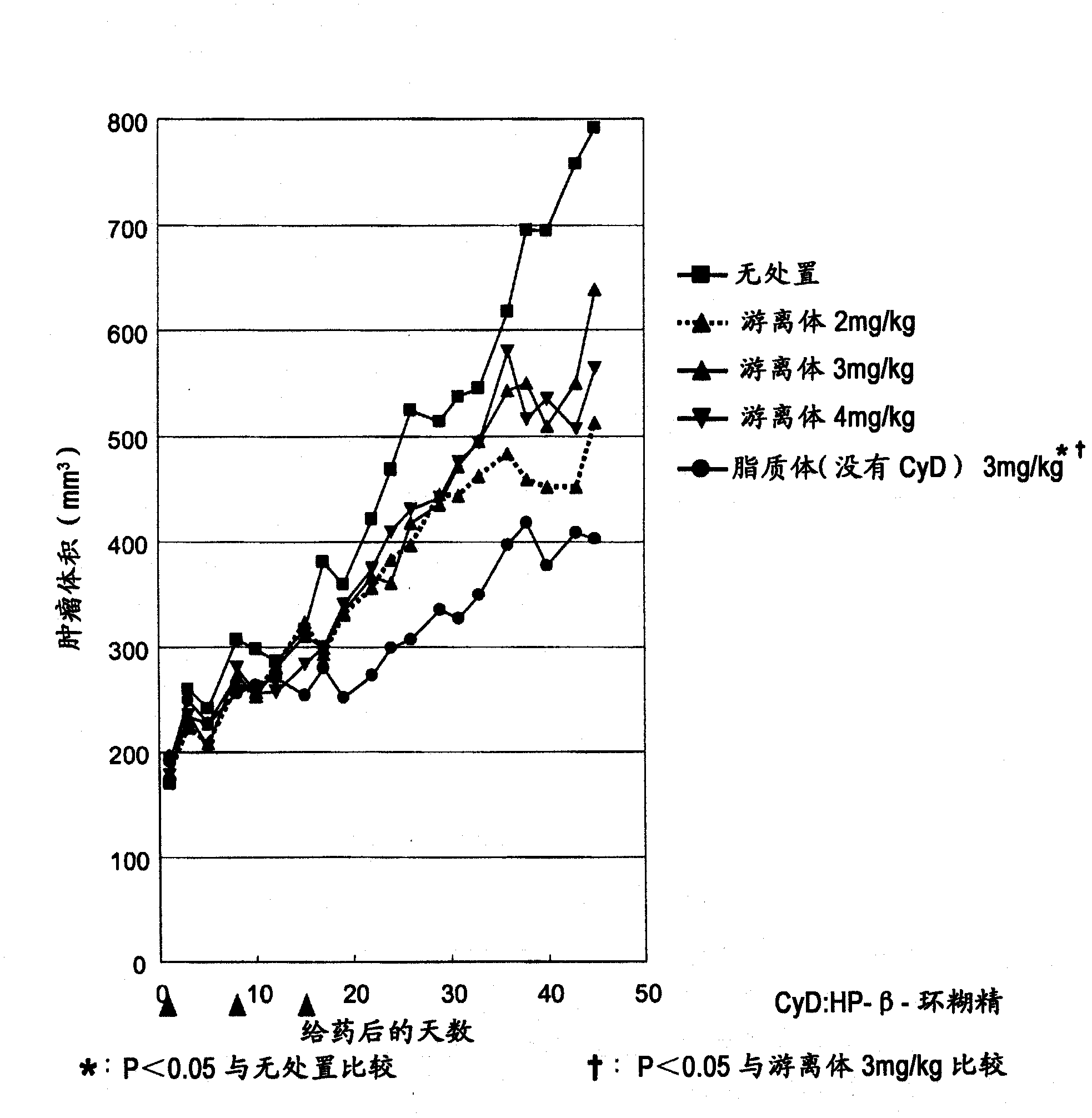
[0206] Weigh 80 mg of the lipid mixture (hydrogenated soybean lecithin: cholesterol: polyethylene glycol 2000-phosphatidylethanolamine = 58.6: 19.2: 22.2 (weight ratio)), and add 2 mL of an aqueous solution for the inner phase of the liposome heated to about 80 ° C. , stir to prepare liposome preparation solution. The liposome preparation solution was sized using an extruder (manufactured by Lipex Biomembranes) heated to about 80° C. to obtain a liposome preparation solution.
[0207]
[0208] The obtained liposome preparation ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com