Application of transition metal oxide
A technology of transition metals and oxides, which is applied in the detection and analysis of biochemical substances, can solve the problem of high detection cost and achieve the effects of high accuracy, good stability and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
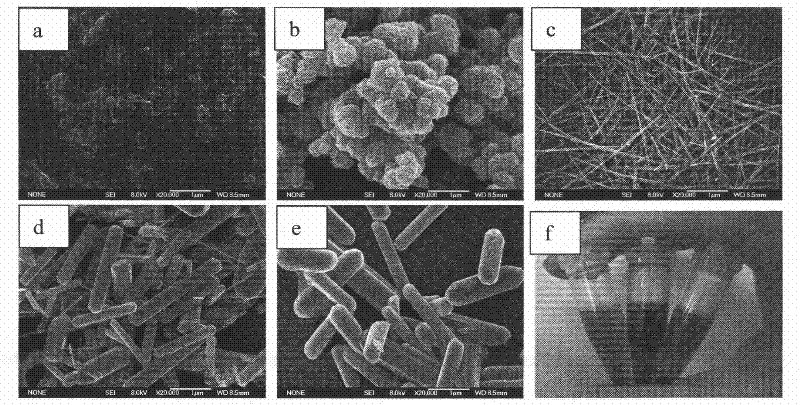
[0020] For the preparation of manganese dioxide nanosheets, refer to related literature reports in J.Am.Chem.Soc.2008, 130, 15938-15943. Specifically: 20 mL loaded with 0.6M TMA and 3% H 2 o 2 To the solution was added 10 ml of 0.3 M manganese chloride solution. The resulting suspension was stirred at room temperature for 12 hours, then centrifuged, washed with Milli-Q water and absolute ethanol, and freeze-dried. Meanwhile, other manganese dioxide nanoparticles, namely nanospheres.
[0021] For manganese dioxide nanoneedles and nanorods, refer to the hydrothermal method reported in J.Cry.Grow.2008, 310, 716-722 for the synthesis method. Specifically: 2 mM potassium permanganate manganese sulfate and 2 mM manganese sulfate were dissolved in Milli-Q water (80 ml). These products were then transferred to a reaction vessel, sealed, and heated at 160°C for a hydrothermal reaction time of 0.5-8 to 72 hours, then centrifuged, washed with Mili-Q water, and finally freeze-dried (s...
Embodiment 2
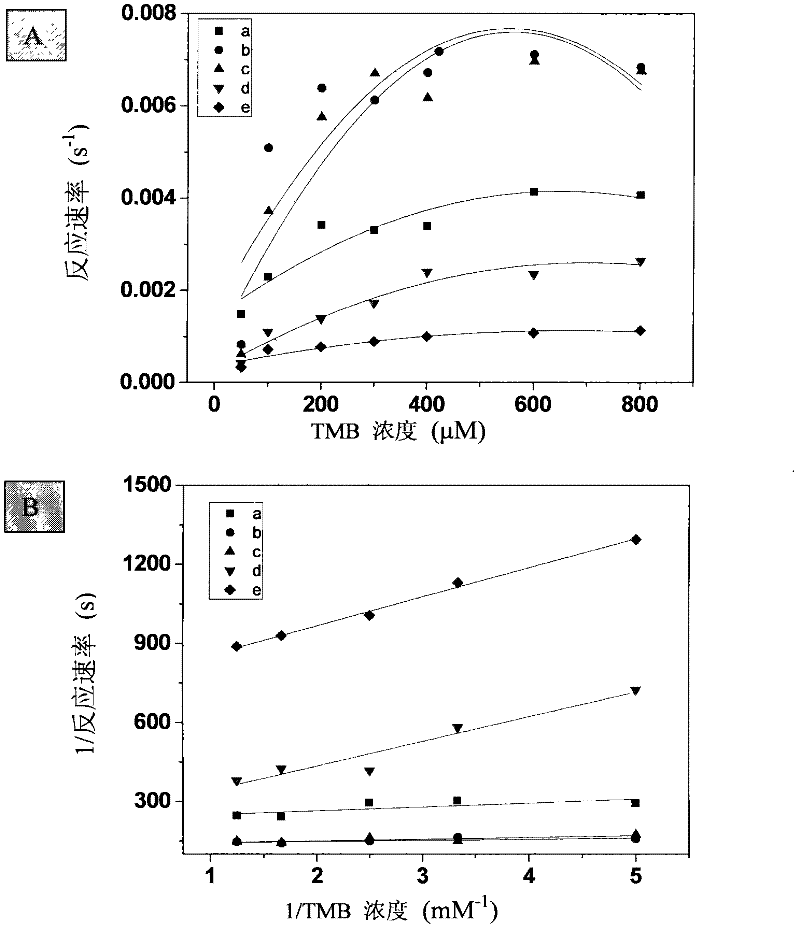
[0030] Take 10 μg each of the manganese oxide nanosheets (a), manganese oxide nanospheres (b), manganese oxide nanowires (c), and manganese oxide nanocomposites (d) obtained in the above examples, respectively, add 50 to 800 μM TMB, and react for 5 minutes. The absorbance of the reaction product was then measured. Draw the curve of TMB concentration and absorbance, and then calculate the kinetic constant (see image 3 ).
[0031] Simultaneously detect the dependency of manganese dioxide nanowire and HRP on pH value (1-12), temperature (5-95 ℃) catalytic activity (see Figure 4 ), studies were carried out using hydrogen peroxide concentrations (0-400 mM). The stability of MnO2 and HRP nanowires at pH and temperature was studied in a series of studies (see Figure 5 ).
[0032] The previously mentioned kinetic experiments were carried out by changing the reaction conditions, and the reaction rate mechanism was studied from 50 to 800 μM TMB in the absence of hydrogen peroxide...
Embodiment 3
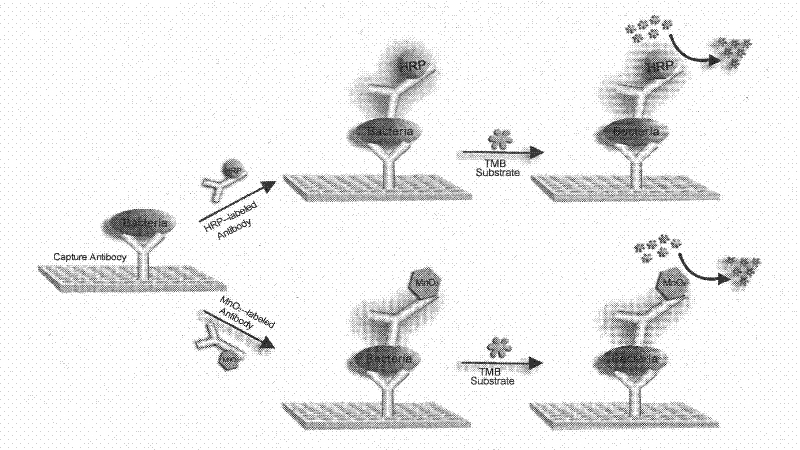
[0034] Anti-sulfate-reducing bacteria antibody (0.5mg mL -1 ) into the wells of the above polystyrene multiwell plate and incubated overnight at 4°C, then added 1% BSA to each well to block non-specific sites and washed three times with PBS (pH 7.4); finally added dropwise to each well Different dilutions of SRB were incubated in 96-well plates for two hours (from 1.8×10 1 cfu mL -1 to 1.8×10 8 cfu mL -1 ), then add HRP-labeled rabbit anti-mouse immunoglobulin (Wuhan Boster Biological Engineering Co., Ltd.) or manganese dioxide-labeled anti-sulfate-reducing bacteria antibody (0.1mg mL -1 ), and incubated for 1 hour. Finally, for the determination of the surface activity of bacterial cells in which the horseradish peroxidase-labeled secondary antibody or manganese dioxide-labeled antibody binds to peroxidase, a substrate solution (50 μM TMB, pH 5.1). The manganese dioxide nanomaterial samples obtained in the above examples were added to the porous plate respectively, and 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com