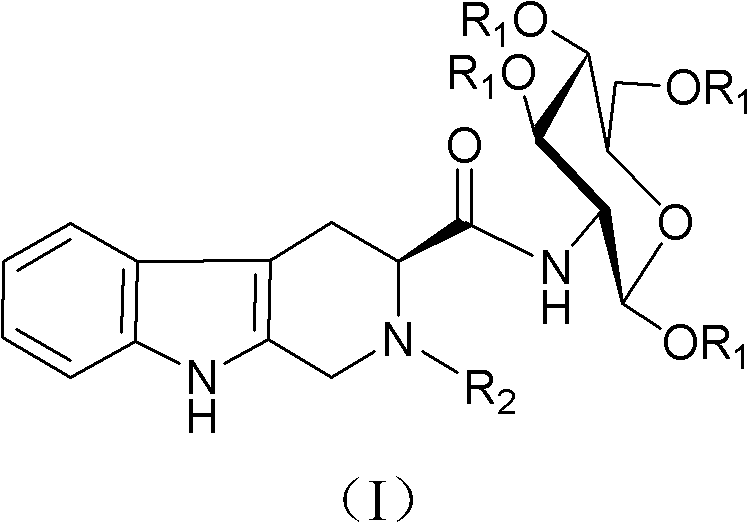
Tetrahydro-beta-carboline derivative, preparation method thereof and use thereof
A technology of tetrahydrocarboline and compound, applied in the field of tetrahydro-β-carboline derivatives, can solve the problems of limited application, poor bioavailability, poor solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
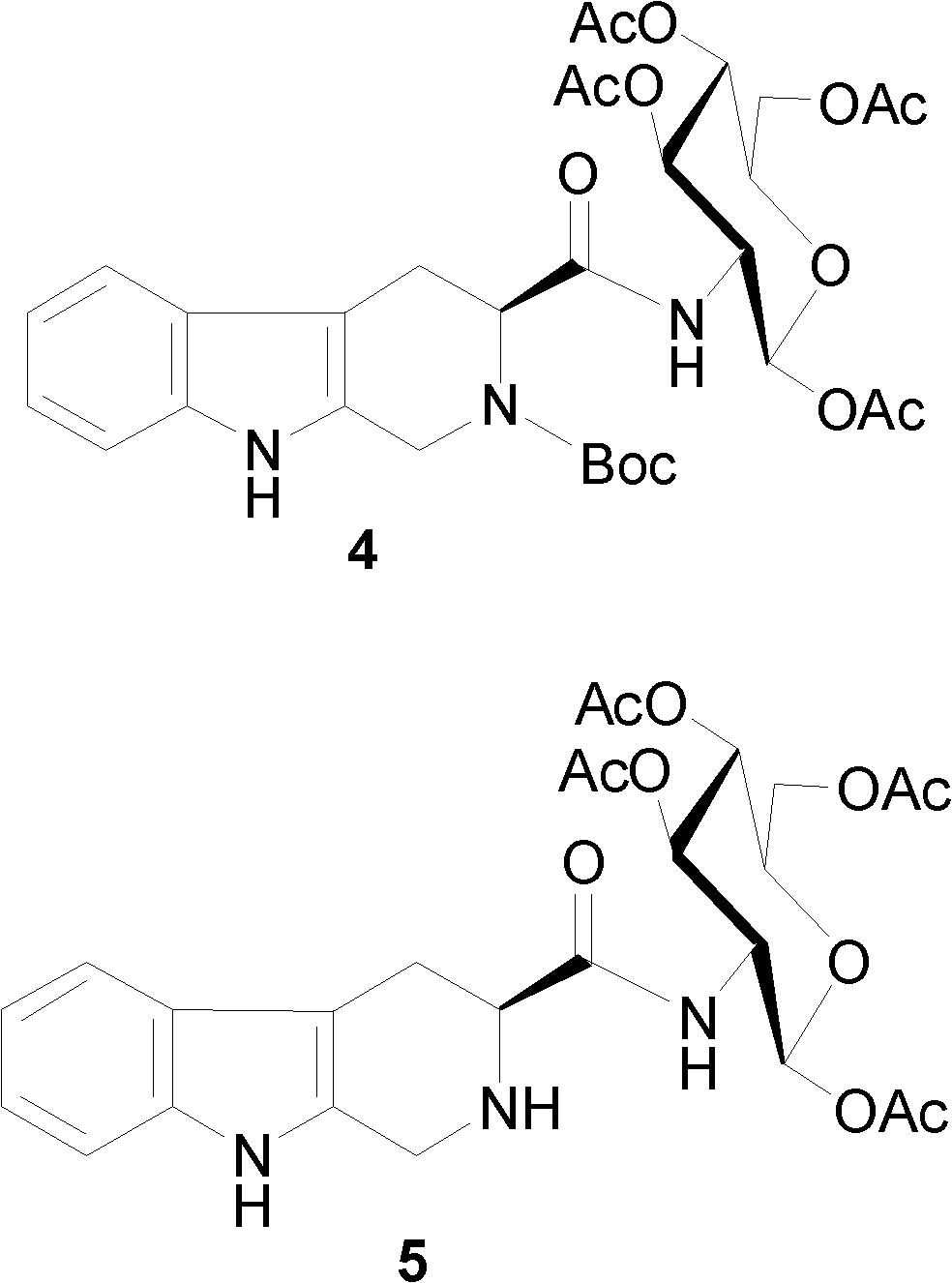
[0029] Example 1 Preparation of N-tert-butoxycarbonyl-tetrahydrocarboline-2-amino-tetraacetyl-D-glucose 4
[0030]
[0031] Under ice bath, N-Boc-tetrahydrocarboline-3-carboxylic acid 3 (3.48g, 11.0mmol) was dissolved in anhydrous THF (150ml), and HOBt (1.62g, 12.0mmol), DCC (2.47g, 12.0mmol) was activated to obtain liquid A. 2-Amino-tetraacetyl-D-glucose (4.45 g, 10.0 mmol) was dissolved in anhydrous THF, and NMM was added to adjust the pH value to 7 to obtain liquid B. Add B to A, adjust the pH to 8, and stir at room temperature for 18 hours. TLC showed disappearance of starting material spots. The reaction mixture was filtered, the filtrate was concentrated to dryness under reduced pressure, and the residue was dissolved in ethyl acetate. The resulting solution was sequentially washed with saturated NaHCO 3 Aqueous solution and 5% KHSO 4 Washing with aqueous solution and saturated NaCl aqueous solution. Separate the ethyl acetate layer, anhydrous Na 2 SO 4 After ...
Embodiment 2 4
[0032] Example 2 Preparation of Tetrahydrocarboxyloyl-2-amino-tetraacetyl-D-glucose 5
[0033]
[0034] N-Boc-tetrahydrocarbolinoyl-2-amino-tetraacetyl-D-glucose 4 (645 mg, 1.00 mmol) was dissolved in 5 ml of 4N hydrogen chloride-ethyl acetate solution. The reaction mixture was stirred at 0°C for 1 h, the reaction mixture was concentrated to dryness under reduced pressure, the residue was dissolved in 10 ml of ethyl acetate, and the resulting solution was concentrated to dryness under reduced pressure. This operation was repeated three times to remove free hydrogen chloride. The obtained solid was used in the next reaction immediately without purification.
Embodiment 3
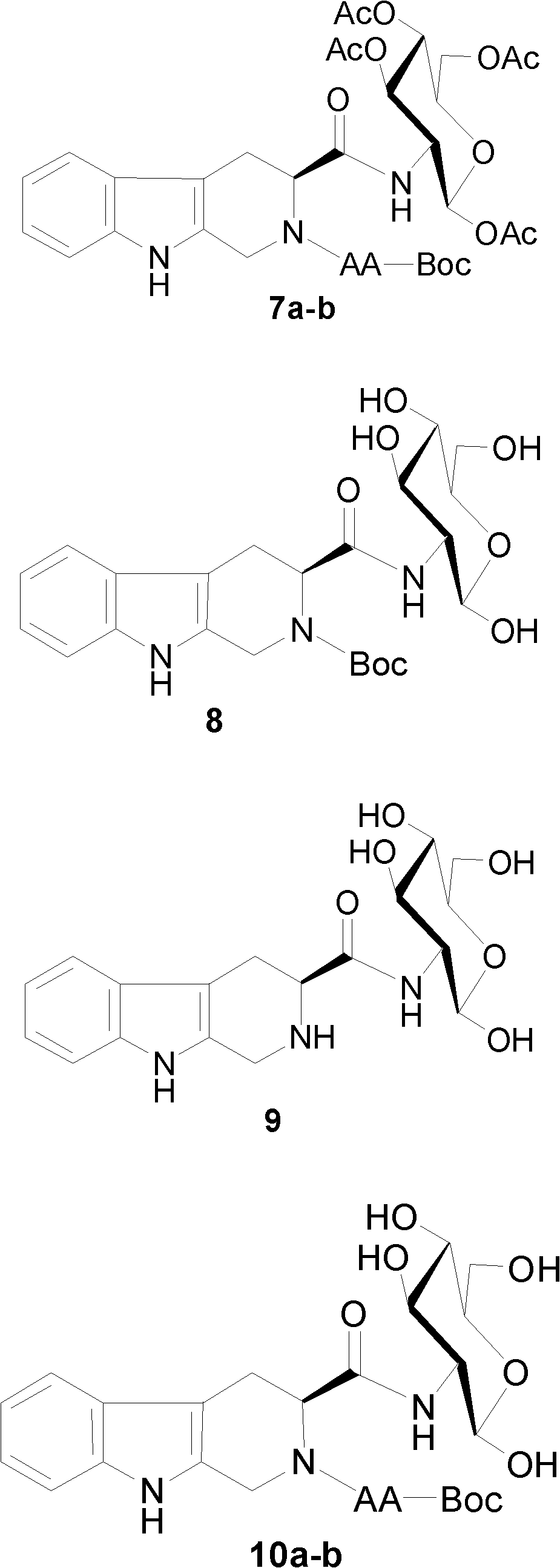
[0035] Example 3 Preparation of N-(Boc-alanyl)-tetrahydrocarbolinoyl-2-amino-tetraacetyl-D-glucose 7a
[0036]
[0037] According to the operation of Example 1, THC-2-amino-tetraacetyl-D-glucose 5 (582mg, 1.00mmol) and Boc-L-Ala-OH 6a (207mg, 1.10mmol), HOBT ( Condensation of 162mg, 1.20mmol) and DCC (247mg, 0.980mmol) in anhydrous THF (10ml) afforded 7a (259mg, 0.362mmol (36%) of the title compound. [α] 25 D -5.8 (c 0.1, CHCl 3 ); IR (cm -1 , KBr, neat): 3381, 1747, 1658; 1 HNMR (300MHz, CDCl 3 ): δ9.41(1H, s, N-H), 8.67(1H, s, N-H), 7.53-7.07(4H, m, Ar-H), 6.21(1H, d, J 2,NH 8.7Hz, N-H), 5.93 (1H, d, J NH,α 5.4Hz, N-H), 5.63 (1H, d, J 1’,2’ 8.7Hz, H-1'), 5.60(1H, m, H-3'), 5.30-5.03(2H, m, H-α, H-2'), 4.96(1H, m, H-3), 4.87-4.74 (2H, m, H-1a, H-1b), 4.36-4.12 (2H, m, H-5', H-6a'), 4.04 (1H, t, J 3’,4’ =J 4’,5’ =12.0Hz, H-4'), 3.74(1H, m, H-6b'), 3.46(1H, m, H-4a), 3.16(1H, m, H-4b), 2.03(3H, s, CH 3 ), 1.99 (3H, s, CH 3 ), 1.95 (3H, s, CH 3 ), 1.58 (3H, d, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com