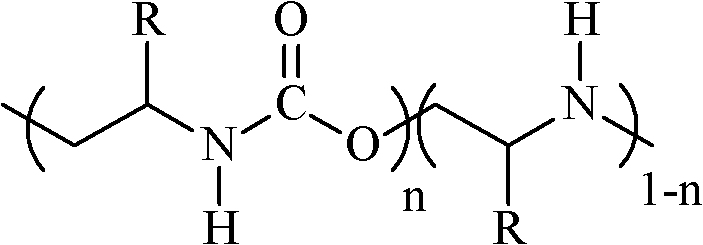
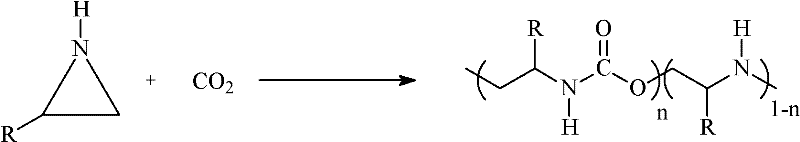
Method for preparing aliphatic polyester (urethane urea-amine)
An aliphatic and urethane technology, applied in the field of polymer material synthesis, can solve the problem that the conversion rate is only 35%
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] From 0.01mol ZnEt 2 Configured and aged rare earth three-way catalyst, the three-way catalyst includes: 0.0005mol yttrium trichloroacetate+20mL 1,3-dioxolane+0.005mol glycerol+0.01mol ZnEt 2 , In CO 2 Add under protection, evacuate at 80℃ to pressure of 20Pa, charge CO 2 Treat for 2h, charge CO 2 10 times, and cooled to room temperature in the autoclave, while adding 80mL 2-methylaziridine, 50mL 1,3-dioxolane into the autoclave, and quickly pass the carbon dioxide pressure regulator to make the carbon dioxide pressure in the autoclave reach 5.0MPa. The polymerization temperature was 70°C, the reaction time was 12 hours, and the stirring speed was 500 rpm. After the polymerization, the autoclave was cooled to room temperature, the residual carbon dioxide in the autoclave was slowly removed, and 100 mL of 5% hydrochloric acid / ethanol solution (V / V) was added to terminate the polymerization and eliminate the catalyst. Ether was slowly added dropwise to the mixture with stir...
Embodiment 2
[0041] From 0.0025mol ZnEt 2 Configured and aged rare earth three-way catalyst, the three-way catalyst includes: 0.000125mol yttrium trichloroacetate+5mL 1,3-dioxolane+0.00125mol glycerol+0.0025mol ZnEt 2 , In CO 2 Add under protection, evacuate 40Pa at 80℃, charge CO 2 Treat for 2h, charge CO 2 10 times, and cooled to room temperature in the autoclave, while adding 20mL 2-methylaziridine, 50mL N,N-dimethylacetamide into the autoclave, quickly through the carbon dioxide pressure regulator to make the pressure of carbon dioxide in the autoclave It reaches 5.0MPa. The polymerization temperature was 80°C, the reaction time was 10 hours, and the stirring speed was 500 rpm. The post-treatment is the same as in Example 1, to obtain 24 g of polymer, and the catalyst efficiency is 1.9×10 5 g / mol Yttrium trichloroacetate. The elemental analysis results were similar to those of Example 1. The polymer alternating structure (urethane content) content was 84%, the carbon dioxide fixation ra...
Embodiment 3
[0043] From 0.01mol ZnEt 2 A rare earth three-way catalyst configured and aged, the three-way catalyst comprising: 0.0005mol neodymium trichloroacetate+20mL 1,3-dioxolane+0.005mol glycerol+0.01mol ZnEt 2 , In CO 2 Add under protection, evacuate 35Pa at 80℃, charge CO 2 Treat for 2h, charge CO 2 10 times, and cooled to room temperature in the autoclave, while adding 80mL 2-methylaziridine, 50mL 1,3-dioxolane into the autoclave, and quickly pass the carbon dioxide pressure regulator to make the carbon dioxide pressure in the autoclave reach 4.5MPa. The polymerization temperature was 80°C, the reaction time was 8 hours, and the stirring speed was 500 rpm. The post-treatment is the same as in Example 1, to obtain 95 g of polymer, and the catalyst efficiency is 1.9×10 5 g / mol neodymium trichloroacetate. The elemental analysis results were similar to those of Example 1. The content of the polymer alternating structure (urethane content) was 83%, the carbon dioxide fixation rate was 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com