Dual PI3K and mTOR inhibitor compounds
A compound, CH2 technology, used in organic chemistry, pharmaceutical combinations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
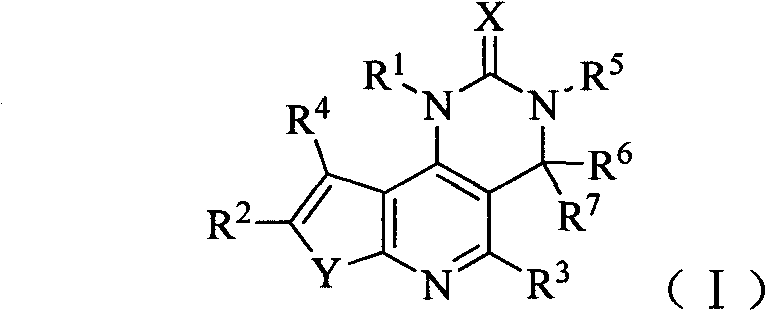
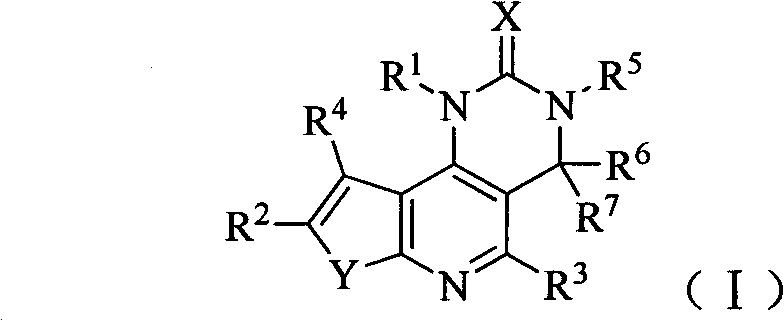
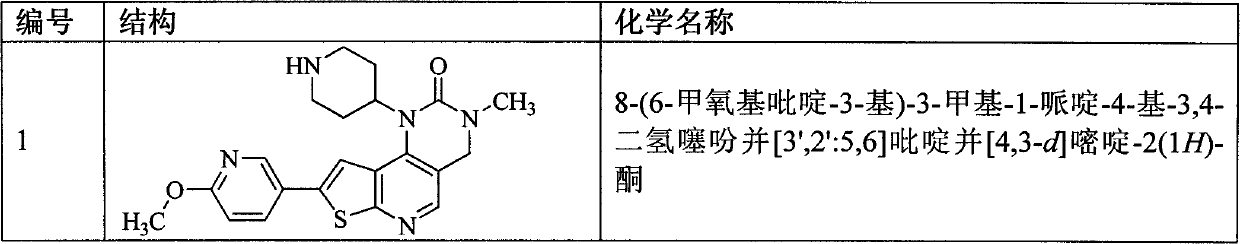
[0157] Example 1 8-(6-methoxypyridin-3-yl)-3-methyl-1-piperidin-4-yl-3,4-dihydrothieno[3′,2′:5,6 ]pyrido[4,3-d] Preparation of pyrimidin-2(1H)-one (compound 1)
[0158] 1. Preparation of tert-butyl thiophen-2-ylcarbamate
[0159]
[0160] 2-thiophene formic acid (10g, 78mmol), DPPA (20.2mL, 93.6mmol) and triethylamine (17.4mL, 125mmol) were dissolved in tert-butanol (150mL), the reaction was refluxed for 6h, cooled, concentrated, and the residue was decanted into saturated NaHCO 3 In the aqueous solution, a pale yellow solid precipitated out, washed three times with water, dried in vacuum at 45°C for 8 hours, and the product was directly used in the next step.
[0161] 2. Preparation of Thiophene-2-amine
[0162]
[0163] The product from the previous step (12 g, 60 mmol) was dissolved in dichloromethane (200 mL), and hydrogen chloride gas was introduced into the mixture for 2 h under ice-bath conditions. TLC (petroleum ether: ethyl acetate = 5: 1) showed that the...
Embodiment 2
[0199] Example 2 (R)-1-[1-(2-hydroxypropionyl)piperidin-4-yl]-8-(6-methoxypyridin-3-yl)-3-methyl-3,4 - dihydrothiazide Preparation of pheno[3′,2′:5,6]pyrido[4,3-d]pyrimidin-2(1H)-one (compound 2)
[0200]
[0201] 8-(6-methoxypyridin-3-yl)-3-methyl-1-piperidin-4-yl-3,4-dihydrothieno[3′,2′:5,6]pyridine And[4,3-d]pyrimidin-2(1H)-one hydrochloride (compound 1) (102mg, 0.23mmol) was dissolved in dichloromethane (20mL), triethylamine (0.05mL) was added respectively, (R )-lactic acid (26.7mg, 0.296mmol), 1-hydroxybenzotriazole (37.8mg, 0.280mmol), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (70.8mg, 0.37mmol), the reaction was stirred at room temperature for 2 hours, TLC (dichloromethane:methanol=10:1) showed that the reaction was complete, the reaction solution was washed with saturated sodium carbonate solution, water, and saturated brine successively, and the organic layer was It was dried with anhydrous sodium sulfate, concentrated, separated and purified...
Embodiment 3
[0204] Example 3 8-(6-methoxypyridin-3-yl)-3-methyl-1-[4-piperazin-1-yl-3-(trifluoromethyl)phenyl]-3,4 - Dihydrothiophene Preparation of [3′,2′:5,6]pyrido[4,3-d]pyrimidin-2(1H)-one (compound 3)
[0205] 1.2-Bromo-4-(4-(4-(tert-butoxycarbonyl)piperazin-1-yl)-3-(trifluoromethyl)aniline)thieno[2,3-b]pyridine-5-carboxylic acid Preparation of ethyl ester
[0206]
[0207] 2-Bromo-4-chlorothieno[2,3-b]pyridine-5-carboxylic acid ethyl ester (see (6) in Example 1) (4.5g, 14mmol) and 4-(4-amino-2 -(trifluoromethyl)phenyl)piperazine-1-carboxylic acid tert-butyl ester (4.0g, 11.6mmol) was dissolved in ethanol (10mL), triethylamine (4.68g, 46.3mmol) was added to the system, and reflux Stir for 48 hours. Cooling, concentration under reduced pressure, column chromatography (petroleum ether: ethyl acetate = 3: 1) gave 2.1 g of light yellow solid, yield 23.8%.
[0208] 2.4-(4-(4-(tert-butoxycarbonyl)piperazin-1-yl)-3-(trifluoromethyl)phenylamino)-2-(6-methoxypyridin-3-yl)thiophene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com