Lanthanum carbonate hydrate, preparation method thereof and method for judging whether alkali lanthanum carbonate is doped in lanthanum carbonate
A technology of lanthanum carbonate hydrate, lanthanum carbonate, applied in chemical instruments and methods, rare earth metal compounds, material analysis using radiation diffraction, etc. and other problems, to achieve the effect of simple operation, accurate and reliable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
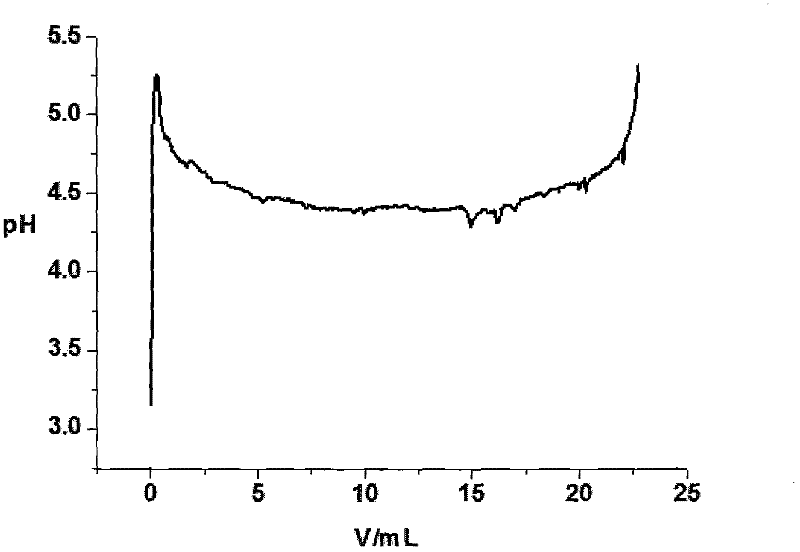
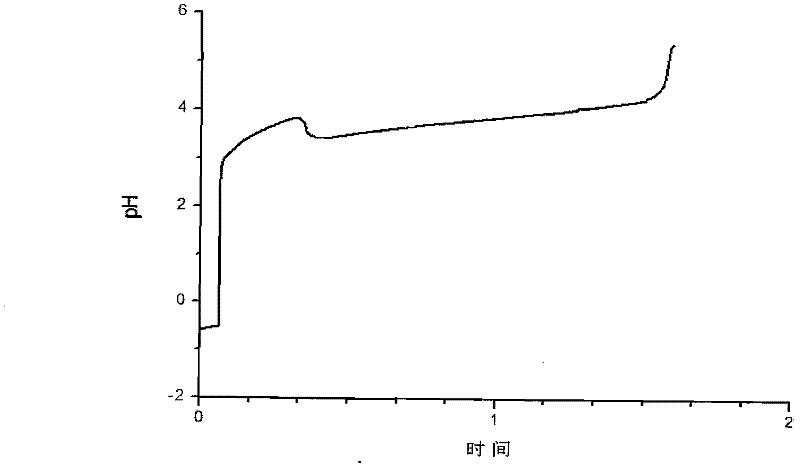
[0078] First add 50mL lanthanum chloride aqueous solution to the flask, its La 3+ The concentration is 2mol / L, and the pH is 0-2. At the beginning, first quickly add 10 mL of sodium bicarbonate aqueous solution to the aqueous solution of lanthanum chloride at a time, and its concentration is 1 mol / L. 2 (CO 3 ) 3 precipitation. Then add sodium bicarbonate solution to the lanthanum chloride solution at a speed of 0.08mL / s until the pH value shows an inflection point, and the reaction stops when the pH value rises abruptly, and a total of 320mL of sodium bicarbonate solution is added. figure 2 The time-pH curve of the preparation of lanthanum carbonate octahydrate by the direct method is given.
[0079] The filtrate was suction filtered, washed three times with distilled water, and the filter cake was placed in a ventilated place to dry naturally.
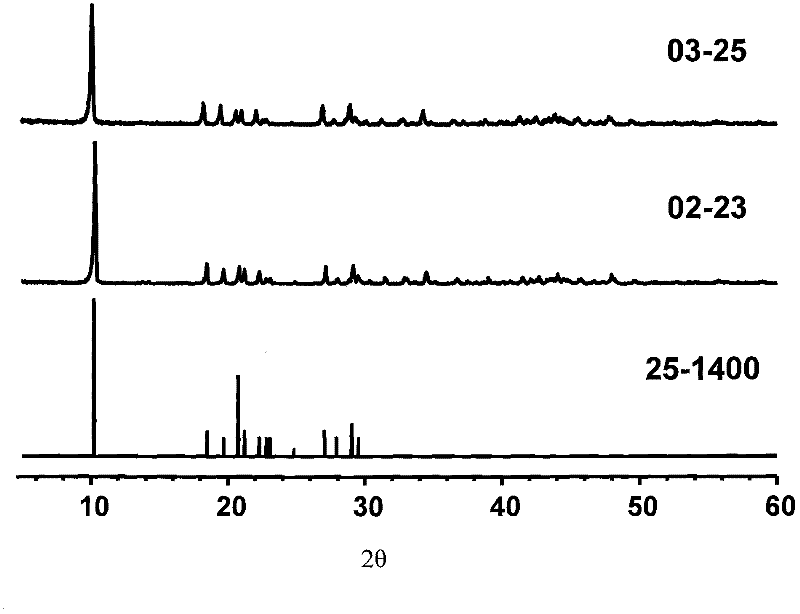
[0080] image 3 A comparison of the obtained lanthanum carbonate octahydrate (labeled 03-25) with the standard card (25-1400)...
Embodiment 2
[0102] First add 50mL lanthanum chloride aqueous solution to the flask, its La 3+ The concentration is 2.5mol / L and the pH is 1.5. At the beginning, first quickly add 15mL sodium bicarbonate aqueous solution to the lanthanum chloride aqueous solution at a time, and its concentration is 0.81mol / L. At this time, the pH value of the solution rises to about 3. 2 (CO 3 ) 3 precipitation. Then add sodium bicarbonate solution to the lanthanum chloride solution at a rate of 2 mL / s until the pH value shows an inflection point, and the reaction stops when the pH value rises abruptly, and a total of 380 mL of sodium bicarbonate solution is added. The filtrate was suction filtered, washed three times with distilled water, and the filter cake was placed in a ventilated place to dry naturally.
[0103] image 3 A comparison of the obtained lanthanum carbonate octahydrate (labeled 02-23) with standard cards (25-1400) is given. It can be seen that the peak positions are basically the sa...
Embodiment 3
[0110] First add 50mL lanthanum chloride aqueous solution to the flask, its La 3+ The concentration is 1.5mol / L, and the pH is 1.0. At the beginning, first quickly add 8 mL of sodium bicarbonate aqueous solution to the aqueous solution of lanthanum chloride at a time, and its concentration is 1.51 mol / L. 2 (CO 3 ) 3 precipitation. Then add sodium bicarbonate solution to the lanthanum chloride solution at a rate of 1.5 mL / s until the pH value shows an inflection point, and the reaction stops when the pH value rises abruptly, and 260 mL of sodium bicarbonate solution is added in total. The filtrate was suction filtered, washed three times with distilled water, and the filter cake was placed in a ventilated place to dry naturally.
[0111] Put the obtained lanthanum carbonate octahydrate in an oven, keep it at a certain temperature under normal pressure for 20 hours, and obtain lanthanum carbonate with 3 to 4 water, specifically, when the temperature is 50°C, you can get The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com