Electron transport material for organic electroluminescent devices and preparation method of electron transport material
An electron transport material and electroluminescence device technology, applied in the field of electron transport materials and their preparation, can solve the problems of single variety of electron transport materials, difficult to optimize performance, difficult structural design, etc., and achieve high reaction yield and short synthesis route. , material structure design and performance optimization easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
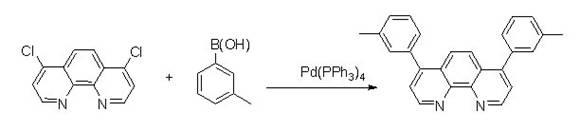
[0035] Weigh 4,7-dichloro-1,10-phenanthroline (2.49g, 10mmol) and dissolve it in 14mL toluene, pour it into the reactor, then add m-tolylboronic acid (2.85g, 24mmol ), Pd(PPh 3 ) 4 (231mg, 0.2mmol), Na 2 CO 3 (2.1 g, 20 mmol) and 7 mL of water. The above mixture was reacted at 90° C. for 24 hours under the protection of argon. Add water to the reaction solution to terminate the reaction, extract with ethyl acetate, wash the organic layer obtained by the extraction with saturated brine first, then wash with pure water, and finally dry, filter, and spin-dry with a rotary evaporator to obtain the crude product, which is then via CH 2 Cl 2 / THF (5:1) was used as mobile phase column chromatography to obtain 3.00 g of analytically pure final product, yield: 76%. 1 H NMR (400 MHz, CDCl3): δ 9.22 (2H, d, J=4.4 Hz), 7.86 (2H, s), 7.57 (2H, d, J=4.4 Hz), 7.43-7.34 (2H, m), 7.33-7.29 (6H, m), 2.44 (6H, s). The reaction equation of the present embodime...
Embodiment 2
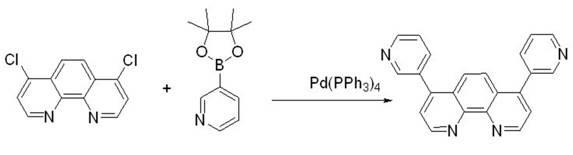
[0039] Weigh 4,7-dichloro-1,10-phenanthroline (2.49g, 10mmol) and dissolve it in 14mL toluene, pour it into the reactor, then add p-phenylboronic acid (4.75g, 24mmol ), Pd(PPh 3 ) 4 (231mg, 0.2mmol), Na 2 CO 3 (2.1 g, 20 mmol) and 7 mL of water. The above mixture was reacted at 90° C. for 24 hours under the protection of argon. Add water to the reaction solution to terminate the reaction, extract with ethyl acetate, wash the organic layer obtained by the extraction with saturated brine first, then wash with pure water, and finally dry, filter, and spin-dry with a rotary evaporator to obtain the crude product, which is then via CH 2 Cl 2 / THF (5:1) was used as mobile phase column chromatography to obtain 3.00 g of analytically pure final product, yield: 76%. 1 H NMR (400 MHz, CDCl3): δ 9.27 (2H, d, J=4.8 Hz), 7.97 (2H, s), 7.77 (4H, m, J=4.8 Hz), 7.69 (4H, m, J=7.6 Hz), 7.65-7.62 (6H, m), 7.52-7.48 (4H, m), 7.42-7.39 (2H, m). The reaction eq...
Embodiment 3
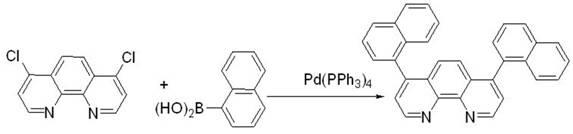
[0043] Weigh 4,7-dichloro-1,10-phenanthroline (2.49g, 10mmol) and dissolve it in 14mL toluene, pour it into the reactor, then add 1-naphthylphenylboronic acid (4.13g, 24mmol), Pd(PPh 3 ) 4 (231mg, 0.2mmol), Na 2 CO 3 (2.1 g, 20 mmol) and 7 mL of water. The above mixture was reacted at 90° C. for 24 hours under the protection of argon. Add water to the reaction solution to terminate the reaction, extract with ethyl acetate, wash the organic layer obtained by the extraction with saturated brine first, then wash with pure water, and finally dry, filter, and spin-dry with a rotary evaporator to obtain the crude product, which is then via CH 2 Cl 2 / THF (5:1) was used as mobile phase column chromatography to obtain 2.6 g of analytically pure final product, yield: 60%. 1 H NMR (400 MHz, CDCl 3 ): δ 9.35 (2H, d, J=4.4 Hz), 7.95-7.89 (4H, m), 7.66 (2H, d, J=4.8 Hz), 7.57-7.53 (2H, m), 7.48-7.43 (4H , m), 7.37-7.29 (4H, m), 7.21 (1H, s), 7.19 (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com