Method for preparing 1,4-stereoregular polytriazoles catalyzed by a ruthenium complex
A technology of stereoregular, ruthenium complexes, applied in the fields of polymer chemistry and materials science, can solve problems such as inability to provide, and achieve the effects of mild conditions, high polymerization efficiency and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
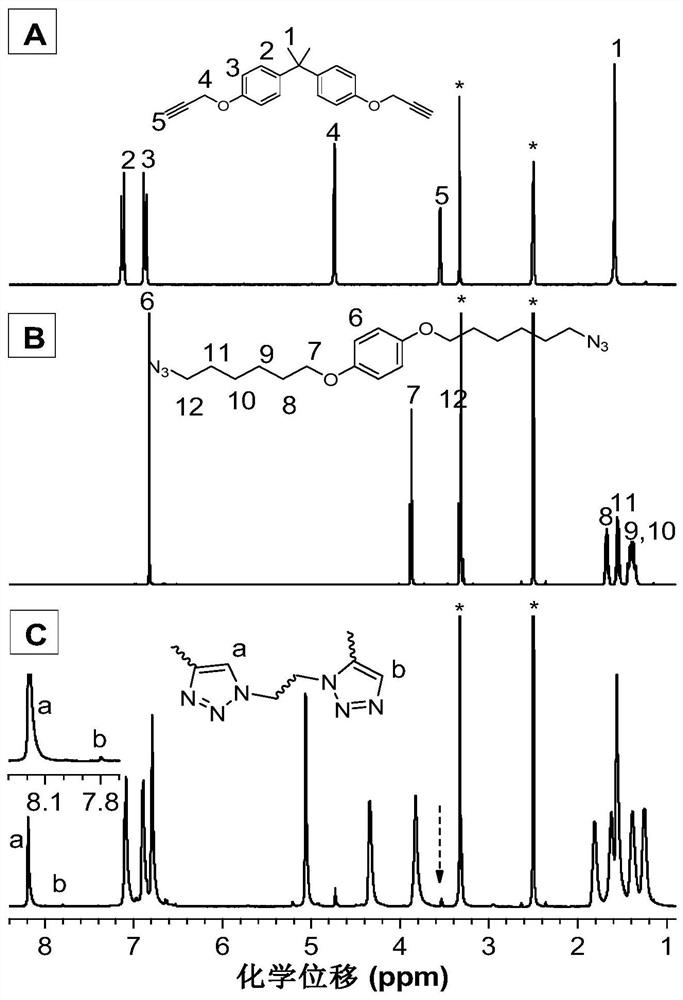
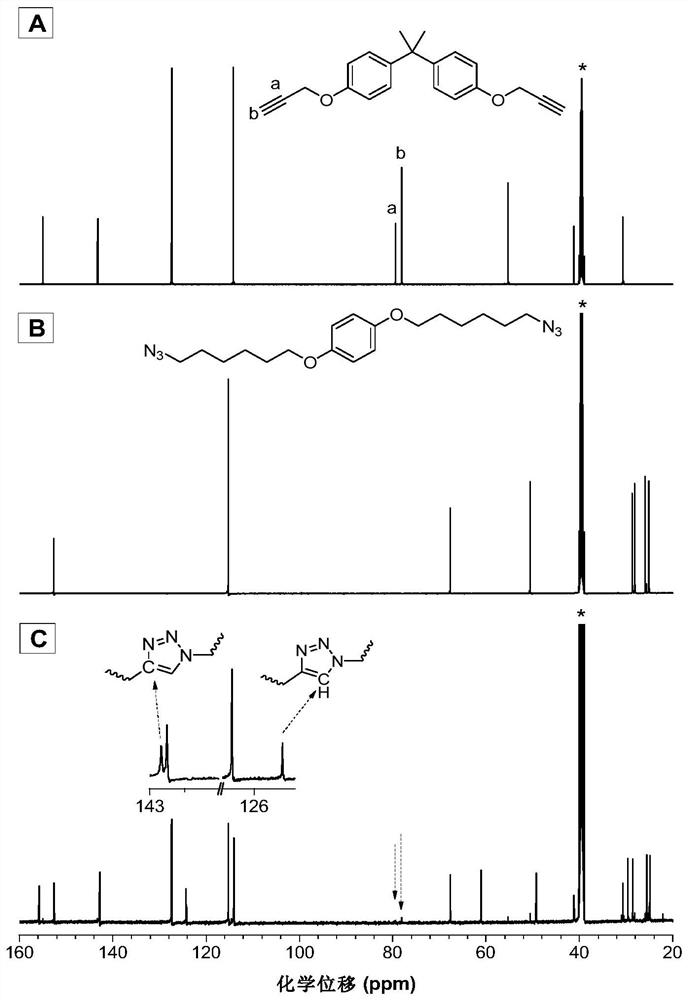
[0041] Ruthenium complex catalyzed M1 and M2 to prepare polytriazole P1:
[0042]
[0043] Wherein, the monomer M1 is synthesized according to the synthesis method in the published literature (A recyclable and reusable supported Cu(I)catalyzed azide-alkyne click polymerization.Sci.Rep.2014,4,5107); the monomer M2 is synthesized according to the published It was synthesized by the synthesis method in the literature (Hyperbranched polytriazoles: Clickpolymerization, regioisomeric structure, light emission, and fluorescent patterning. Macromolecules 2008, 41, 3808-3822).
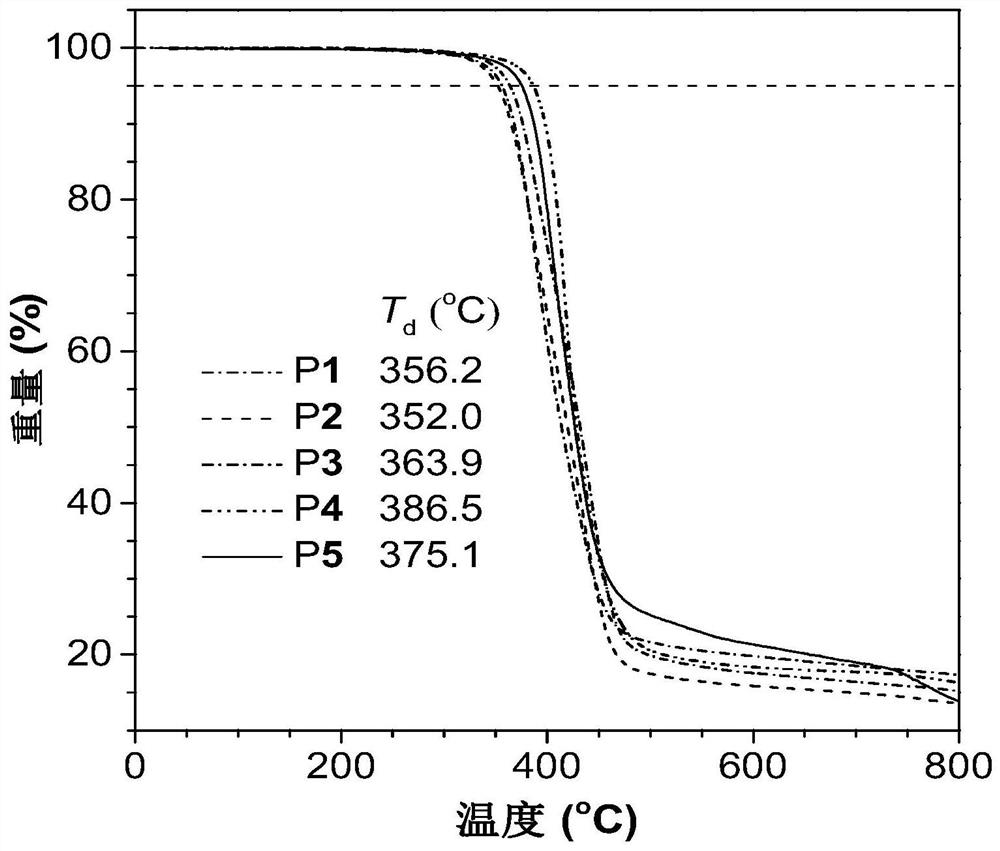
[0044] Add 152mg (0.5mmol) of monomer M1, 180mg (0.5mmol) of monomer M2 and 9.2mg (0.01mmol) of tris(triphenylphosphine)carbonyl dihydroruthenium(II) into a 10mL polymerization tube, vacuumize and change nitrogen Three times, use a syringe to inject 1 mL of ultra-dry N,N-dimethylformamide. After the monomer is completely dissolved, put it into an oil bath at a constant temperature of 80°C and react for 5 hou...
Embodiment 2
[0049] Ruthenium complex catalyzed M1 and M3 to prepare polytriazole P2:
[0050]
[0051] Among them, the monomers M1 and M3 were synthesized according to the synthesis method in the published literature (A recyclable and reusable supported Cu(I) catalyzed azide-alkyne click polymerization. Sci. Rep. 2014, 4, 5107).
[0052] Add 152mg (0.5mmol) of monomer M1, 239mg (0.5mmol) of monomer M3 and 9.2mg (0.01mmol) of tris(triphenylphosphine)carbonyl dihydrogenruthenium(II) into a 10mL polymerization tube, vacuumize and change nitrogen Three times, use a syringe to inject 1 mL of ultra-dry N,N-dimethylformamide. After the monomer is completely dissolved, put it into an oil bath at a constant temperature of 80°C and react for 5 hours. After the reaction is over, add 4 mL of chloroform to the polymerization tube for dilution, filter the obtained polymer solution through cotton and drop it into 110 mL of vigorously stirred (750 rpm) n-hexane / chloroform mixture (volume ratio 10:1) ...
Embodiment 3
[0056] Ruthenium complex catalyzed M1 and M4 to prepare polytriazole P3:
[0057]
[0058] Wherein, the monomer M1 is synthesized according to the synthesis method in the published literature (A recyclable and reusable supported Cu(I)catalyzed azide-alkyne click polymerization.Sci.Rep.2014,4,5107); the monomer M4 is synthesized according to the published It was synthesized according to the synthesis method in the literature (Metal-free click polymerization: Synthesis and photonic properties of poly(aroyltriazole)s. Adv. Funct. Mater. 2009, 19, 1891-1900).
[0059] Add 152mg (0.5mmol) of monomer M1, 307mg (0.5mmol) of monomer M4 and 9.2mg (0.01mmol) of tris(triphenylphosphine)carbonyl dihydrogenruthenium(II) into a 10mL polymerization tube, vacuumize and change nitrogen Three times, use a syringe to inject 1 mL of ultra-dry N,N-dimethylformamide. After the monomer is completely dissolved, put it into an oil bath at a constant temperature of 80°C and react for 5 hours. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com