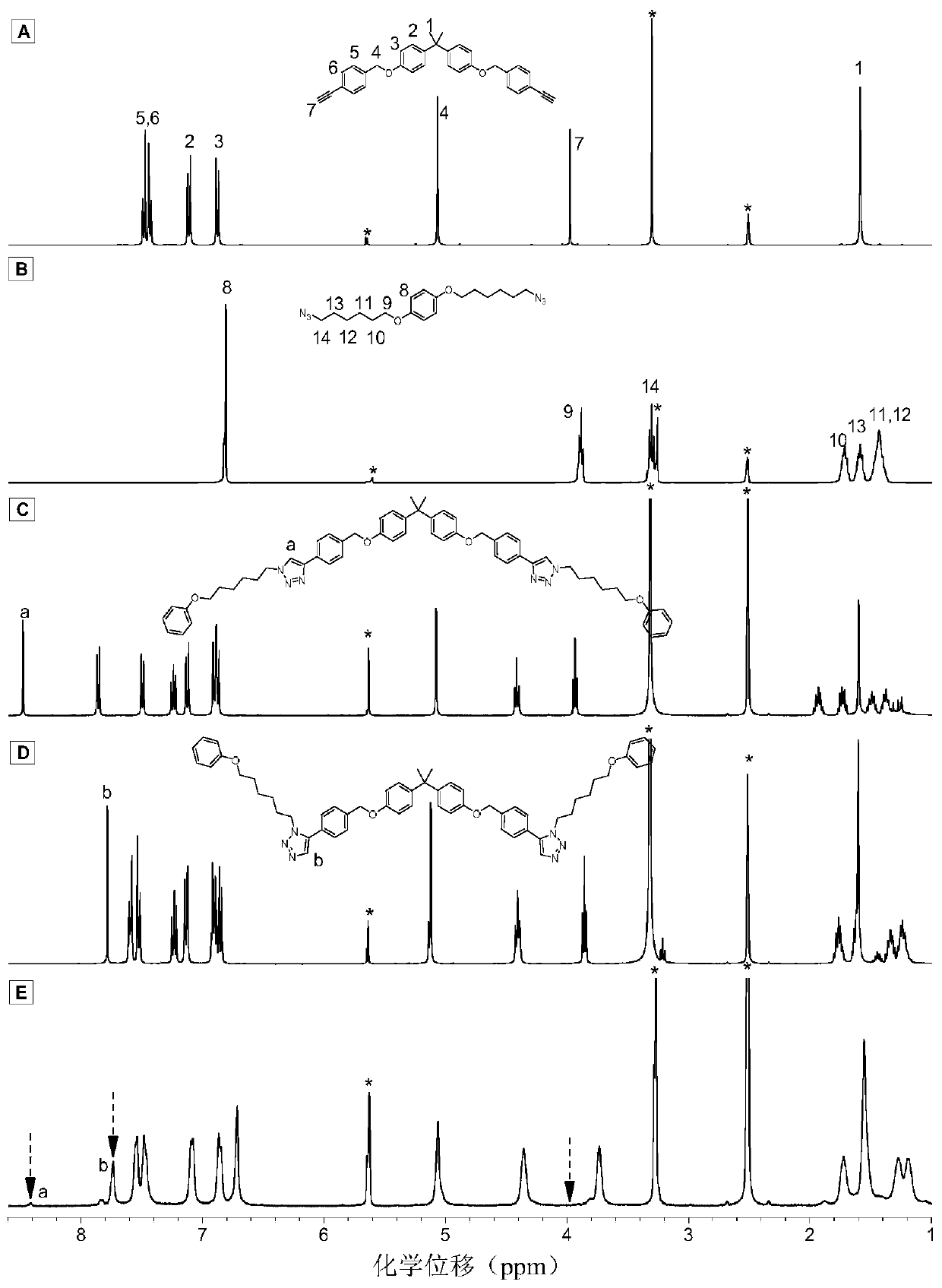
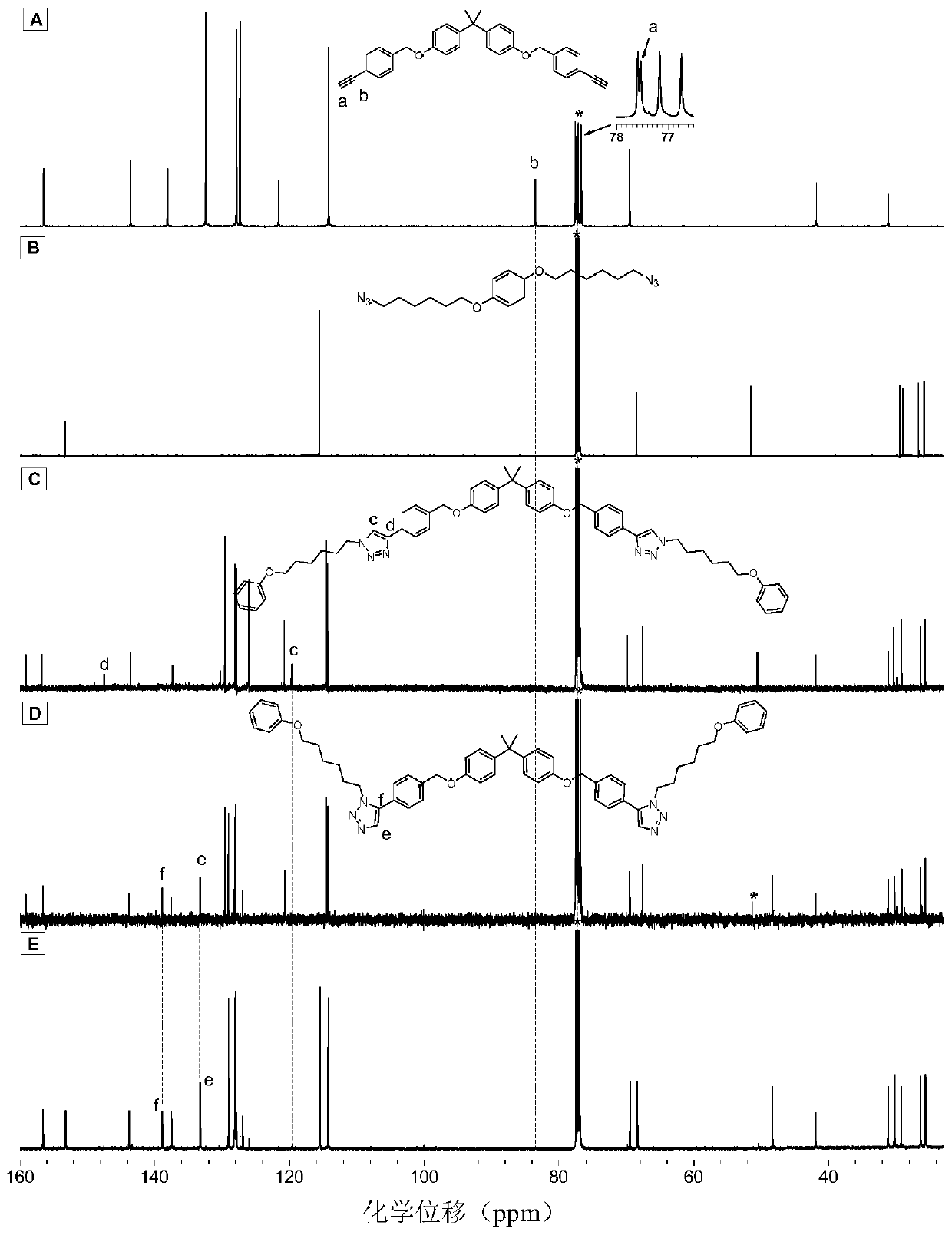
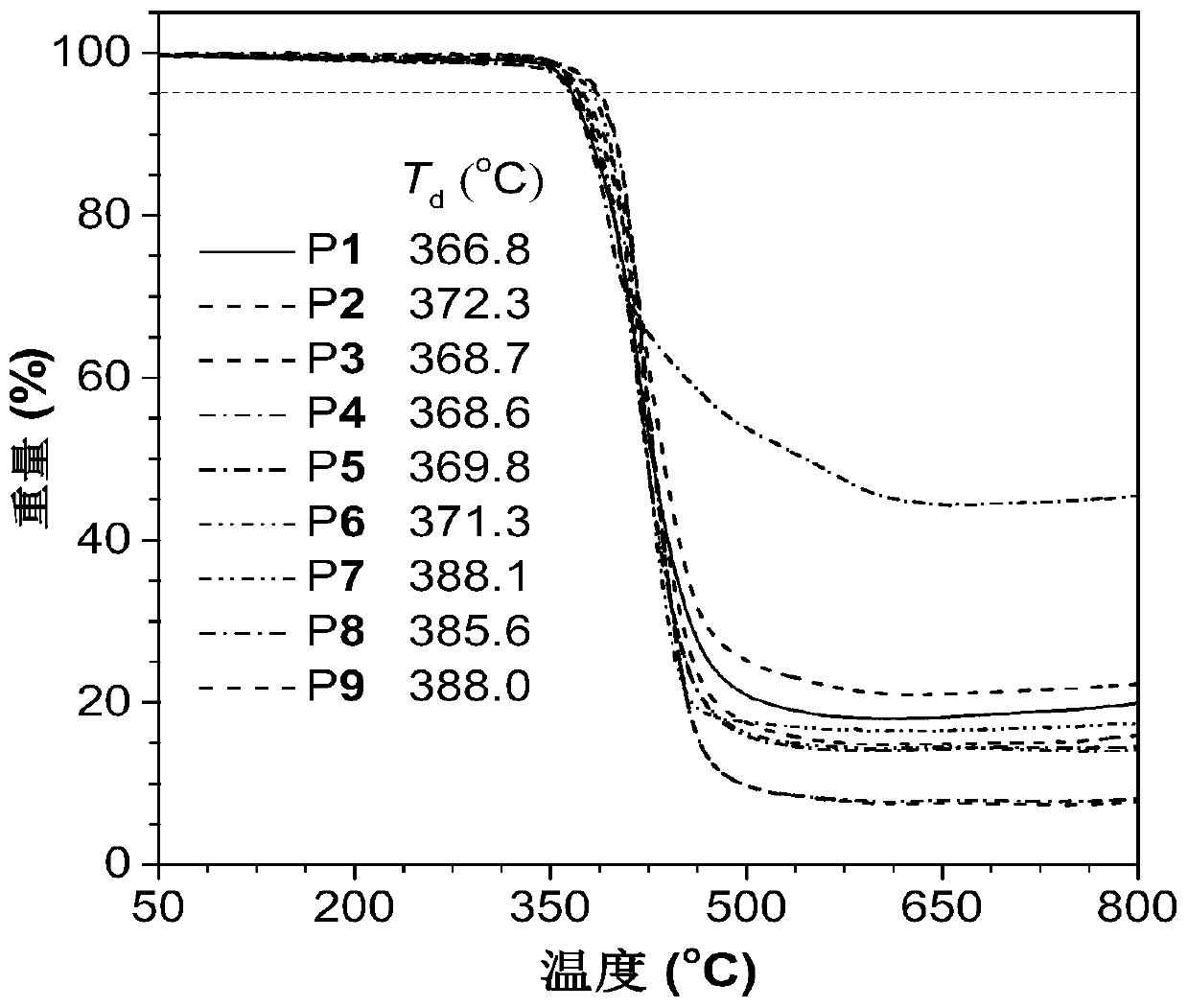
Method for preparing 1,5-stereoregular polytriazole by catalysis of nickel complex
A technology of stereoregular and nickel complexes, applied in the fields of polymer chemistry and materials science, to achieve the effects of high polymerization efficiency, high regioregularity and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Nickel complex catalyzed M1 and M2 to prepare polytriazole P1:
[0045]
[0046] Wherein, the monomer M1 is synthesized according to the synthesis method in the published literature (Catalyst-free thiol-yne clickpolymerization: A powerful and facile tool for preparation of functional poly(vinylene sulfide)s. Macromolecules 2014,47,1325-1333); Monomer M2 was synthesized according to the synthesis method in the published literature (Hyperbranched polytriazoles: Click polymerization, regioisomeric structure, light emission, and fluorescent patterning. Macromolecules 2008, 41, 3808-3822).
[0047]Add 91.2 mg (0.2 mmol) of monomer M1, 72 mg (0.2 mmol) of monomer M2, 7.6 mg (0.04 mmol) of nickelocene, 23.1 mg (0.04 mmol) of 4,5-bisdiphenyl into a 10 mL polymerization tube Phosphine-9,9-dimethylxanthene and 65.2mg (0.2mmol) cesium carbonate, evacuate and change nitrogen for 3 times, inject 0.5mL ultra-dry N,N-dimethylformamide with a syringe, and react at room temperature ...
Embodiment 2
[0057] Nickel complex catalyzed M1 and M3 to prepare polytriazole P2:
[0058]
[0059] Wherein, the monomer M1 is synthesized according to the synthesis method in the published literature (Catalyst-free thiol-yne clickpolymerization: A powerful and facile tool for preparation of functional poly(vinylene sulfide)s. Macromolecules 2014,47,1325-1333); Monomer M3 was synthesized according to the synthesis method in the published literature (A recyclable and reusable supported Cu(I)catalyzed azide-alkyne click polymerization.Sci.Rep.2014,4,5107).
[0060] Add 91.2mg (0.2mmol) of monomer M1, 95.6mg (0.2mmol) of monomer M3, 7.6mg (0.04mmol) of nickelocene, 23.1mg (0.04mmol) of 4,5-diphenyl into a 10mL polymerization tube Phosphine-9,9-dimethylxanthene and 65.2mg (0.2mmol) cesium carbonate, evacuate and change nitrogen for 3 times, inject 0.5mL ultra-dry N,N-dimethylformamide with a syringe, at room temperature React for 30 minutes. After the reaction is over, add 4 mL of dichlo...
Embodiment 3
[0064] Nickel complex catalyzed M1 and M4 to prepare polytriazole P3:
[0065]
[0066] Wherein, the monomer M1 is synthesized according to the synthesis method in the published literature (Catalyst-free thiol-yne clickpolymerization: A powerful and facile tool for preparation of functional poly(vinylene sulfide)s. Macromolecules 2014,47,1325-1333); Monomer M4 was synthesized according to the synthesis method in the published literature (A recyclable and reusable supported Cu(I)catalyzed azide-alkyne click polymerization.Sci.Rep.2014,4,5107).
[0067] Add 91.2mg (0.2mmol) of monomer M1, 122.8mg (0.2mmol) of monomer M4, 7.6mg (0.04mmol) of nickelocene, 23.1mg (0.04mmol) of 4,5-diphenylene into a 10mL polymerization tube Phosphine-9,9-dimethylxanthene and 65.2mg (0.2mmol) cesium carbonate, evacuate and change nitrogen for 3 times, inject 0.5mL ultra-dry N,N-dimethylformamide with a syringe, at room temperature React for 30 minutes. After the reaction is over, add 4 mL of di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com