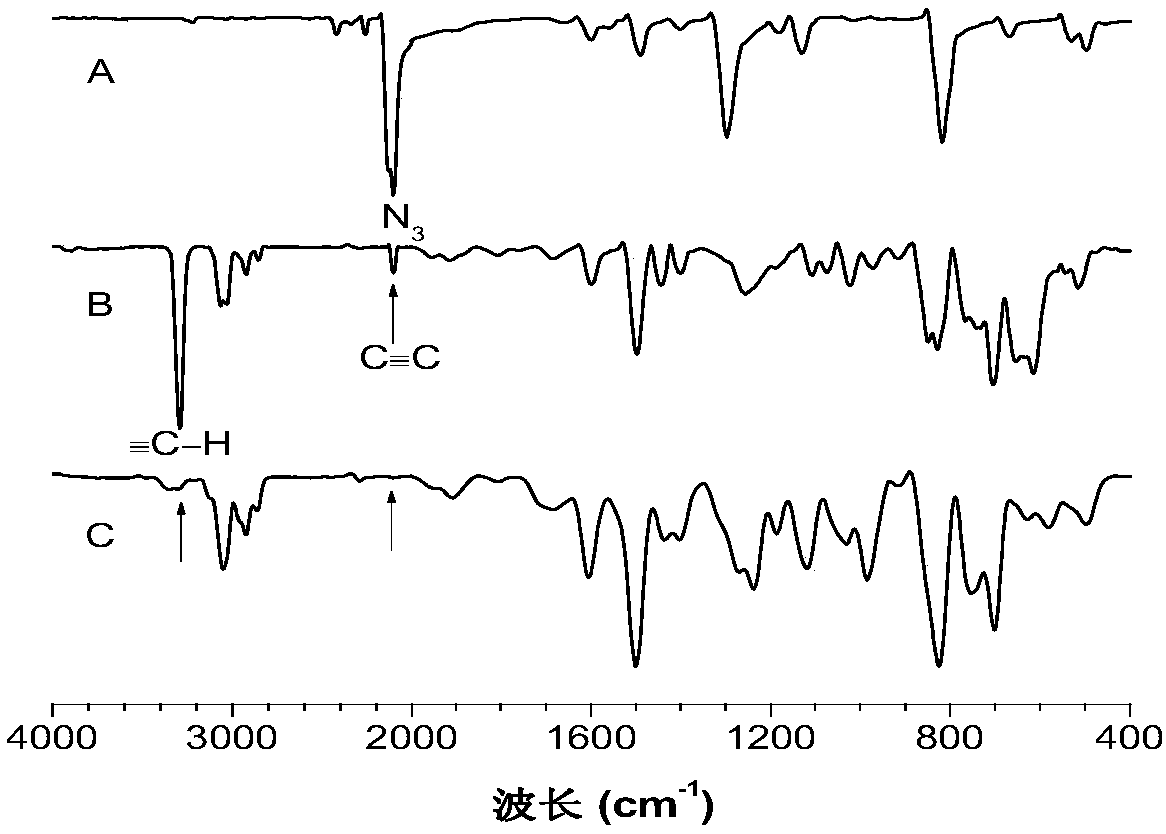
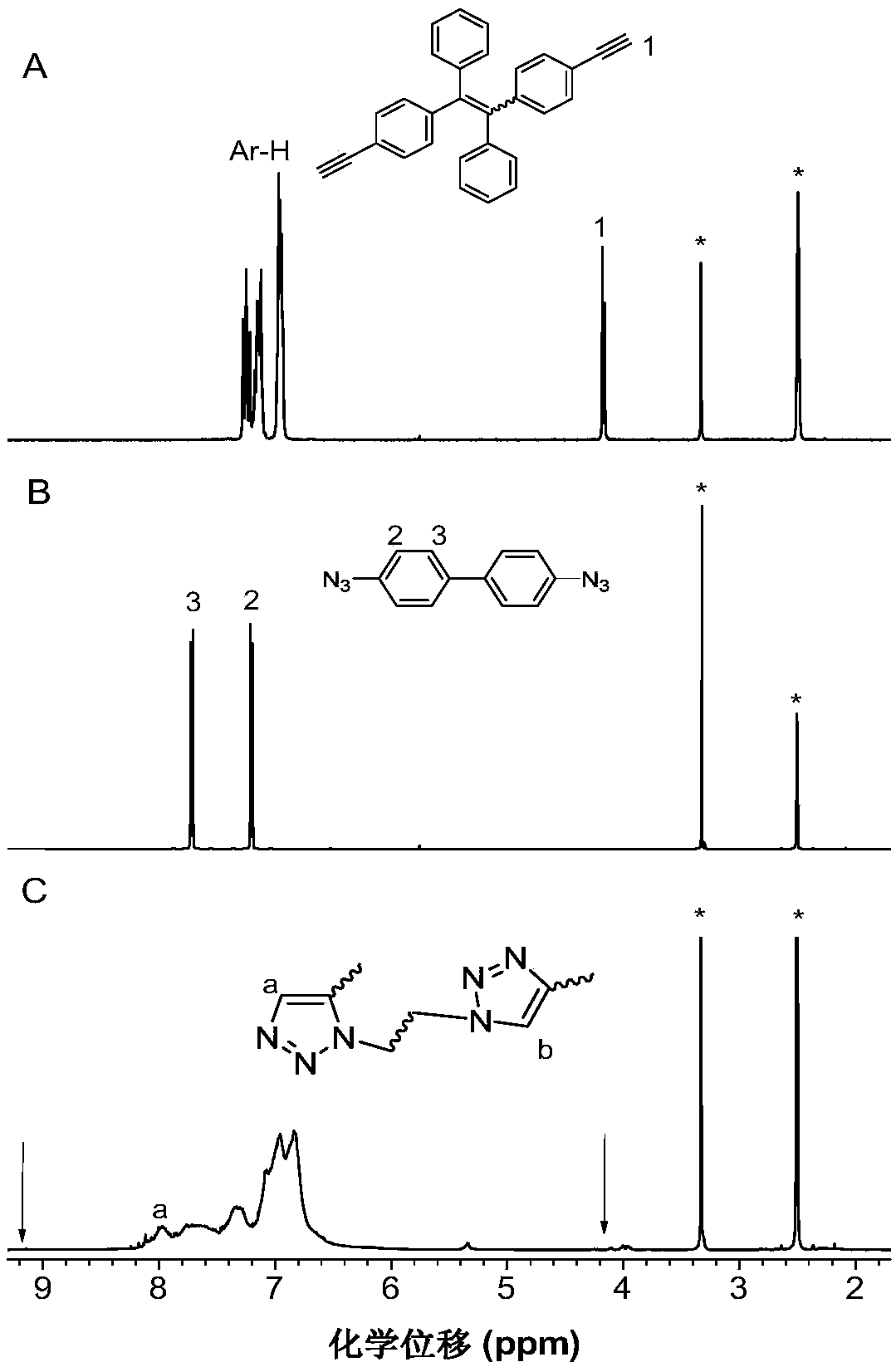
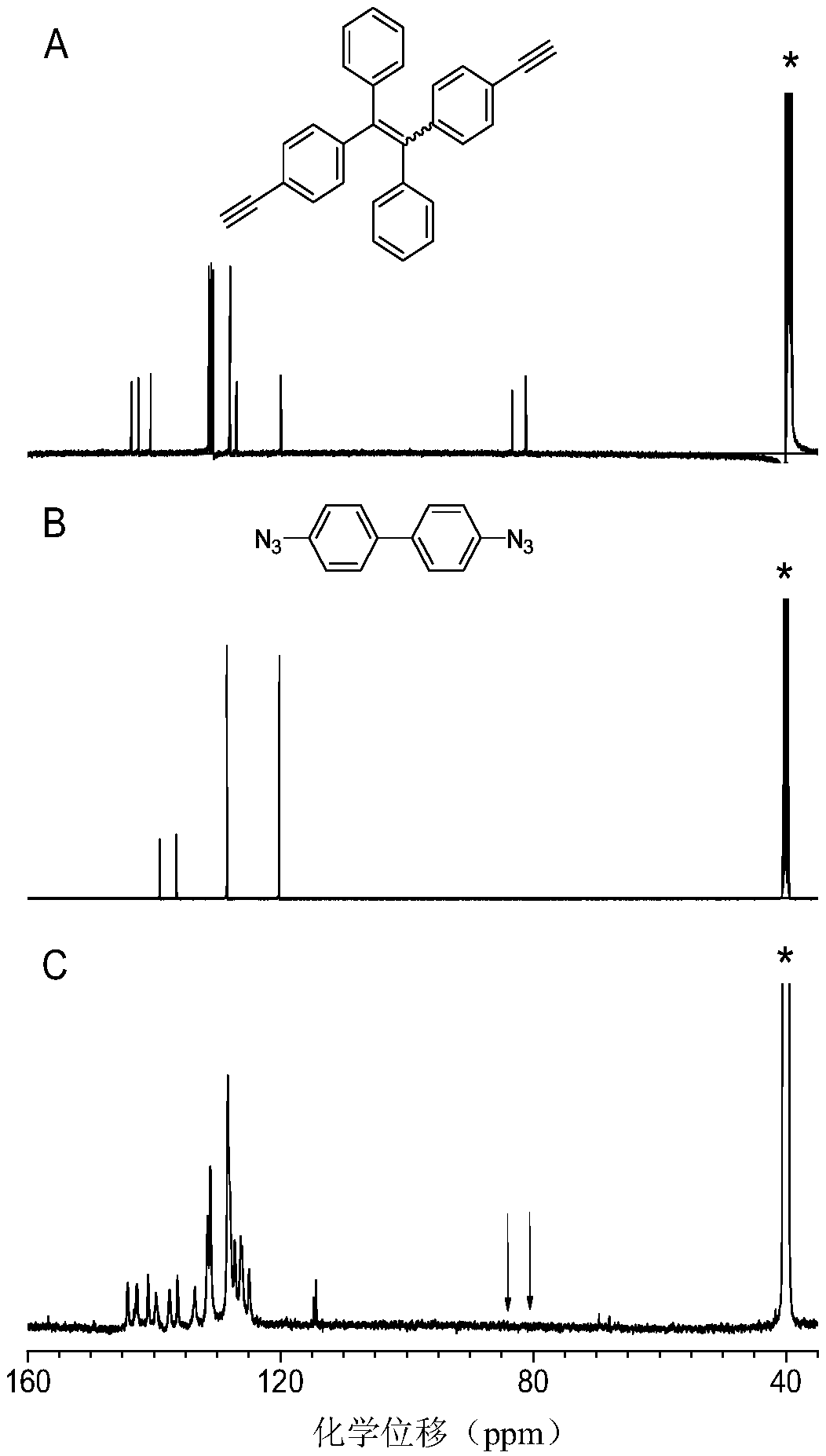
A kind of method for preparing 1,5-stereoregular polytriazole mediated by phosphazene base
A stereoregular, polytriazole technology, applied in the field of polymer preparation, can solve the problems of poor product solubility, difficult metal catalysts, differences in luminescence properties, mechanical properties, crystallization behavior, etc., to achieve high yield, reaction The effect of easy availability of raw materials and less impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This embodiment utilizes phosphazene base t-BuP 4 The preparation of polytriazole (P1) by mediating binary azido compound (1a) and binary alkynyl compound (2a) is as follows:
[0038]
[0039] Wherein, the synthesis method of the binary azido-based compound, that is, the monomer 1a, is according to the published literature (Yuan, W.Z., Mahtab, F., Gong, Y.Y., et al. Synthesis and self-assembly of tetraphenylethene andbiphenyl based AIE-active triazoles [J].J.Mater.Chem., 2012,22,10472) synthetic method; the synthetic method of binary alkynyl compound, namely monomer 2a, is according to the published literature (Yao, B.C., Mei, J., Li, J., etal.Catalyst-Free Thiol–Yne Click Polymerization: A Powerful and FacileTool for Preparation of Functional Poly(vinylene sulfide)s[J].J.Mater.Chem.2011,21,4056; Macromolecule 2014,47 , 1325) synthetic method.
[0040] phosphazene base t-BuP 4 A method for preparing a polytriazole (P1) mediated by a binary azido compound (1a) and ...
Embodiment 2
[0043] This embodiment utilizes phosphazene base t-BuP 4 Mediating binary azido compound (1a) and binary alkynyl compound (2b) to prepare polytriazole P2, the synthetic route is as follows:
[0044]
[0045] The synthetic method of binary azido-based compound, that is, monomer 1a, is according to published literature (Yuan, W.Z., Mahtab, F., Gong, Y.Y., et al. Synthesis and self-assembly of tetraphenyle theneand biphenyl based AIE-active triazoles[ J].J.Mater.Chem., 2012,22,10472) is synthesized by the synthetic method; The synthetic method of binary alkynyl compound is monomer 2a according to published literature (Yao, B.C., Mei, J., Li , J., etal.Catalyst-Free Thiol–Yne Click Polymerization: A Powerful and Facile Tool for Preparation of Functional Poly(vinyle ne sulfide)s[J].J.Mater.Chem.2011,21,4056; Macromolecule 2014, 47,1325) synthetic method.
[0046] phosphazene base t-BuP 4 A method for preparing a polytriazole (P2) by mediating a binary azido compound (1a) and ...
Embodiment 3
[0052] This embodiment utilizes phosphazene base t-BuP 4 Mediating binary azido compound (1a) and binary alkynyl compound (2c) to prepare polytriazole P3, the synthetic route is as follows:
[0053]
[0054] Wherein the synthetic method of binary azido-based compound, that is, monomer 1a, is according to published literature (Yuan, W.Z., Mahtab, F., Gong, Y.Y., et al. Synthesis and self-assembly of tetraphenylethene andbiphenyl based AIE-active triazoles[ J].J.Mater.Chem., 2012,22,10472) synthetic method; the synthetic method of binary alkynyl compound, namely monomer 2c, is according to the published literature (Zhao, E.G., Li, H.K., Wu, H.Q., etal.Structure-dependent emission of polytriazoles[J].Polym.Chem., 2014,5,2301) synthetic method.
[0055] phosphazene base t-BuP 4 The method for preparing polytriazole P3 by mediating a binary azido compound (1a) and a binary alkynyl compound (2c) comprises the following steps:
[0056] Add 23.6 mg (0.1 mmol) of monomer 1a and 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com