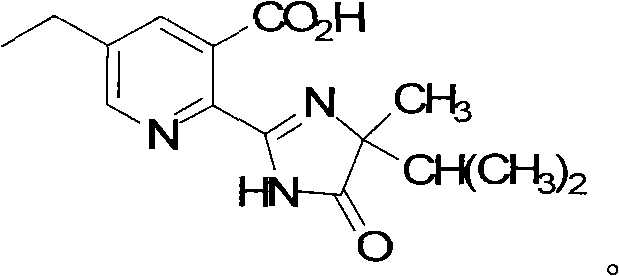
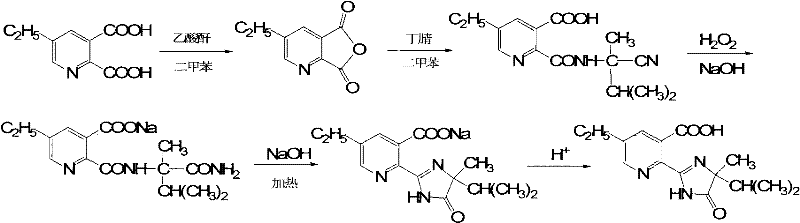
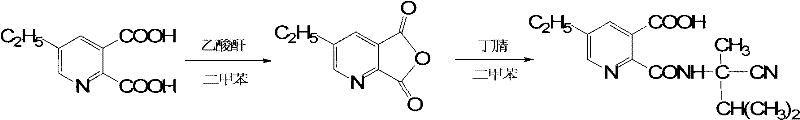
Method for preparing imazethapyr
A technology of imazethapyr and ethyl nicotinic acid, which is applied in the direction of organic chemistry, can solve the problems of uncertain product crystal form and large amount of waste water, and achieve the effects of reducing raw material costs, improving quality, and increasing reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0026] Example 1 Synthesis of 2-[(1-cyano-1,2-dimethylpropyl)-formamido]-5-ethyl nicotinic acid
[0027]
[0028] Add 40.2g (0.2mol, 97%) of 5-ethylpyridinedicarboxylic acid, 23g (0.22mol, 97.6%) of acetic anhydride and 160g of xylene into a 500ml reaction flask, heat up and reflux for 0.5h, then cool down to 25-30°C , dropwise added 26g (0.22mmol, 95%) 2-amino-2,3-dimethylbutyronitrile, after the dropwise addition was completed, it was incubated at 8-12°C for 1h, and the HPLC tracking reaction was complete. After toluene, the temperature was lowered to precipitate a solid, which was filtered and dried to obtain 50.2 g of 2-[(1-cyano-1,2-dimethylpropyl)-carboxamido]-5-ethylnicotinic acid with a content of 97%. The rate is 87.3%.
example 2II
[0029] Example 2 II crystal form imazethapyr
[0030]
[0031]In a 500ml reaction flask, the 50.2g product (content 97%) obtained in Example 1 was dissolved in 106.7g (0.8mol, 30%) of NaOH aqueous solution, stirred for 10min, and 40.8g was added dropwise at room temperature 20-25°C (0.3mol, 25%) hydrogen peroxide, after the dropwise addition, keep warm at 20-25°C for 2h, then raise the temperature to 70°C for 2h, then raise the temperature to 90°C for 20min. Afterwards, cool down to 30°C-40°C, add hydrochloric acid dropwise to pH = 3-4, and keep for 20 minutes; then cool the material to 10°C, keep it warm for 30 minutes, and filter. The filter cake was washed three times with 50 g of water, and dried to obtain 56.2 g of product with a content of 98.7%, a yield of 96%, and a turbidity of 75 NTU.
[0032] MP: 171.4-173.9°C.
[0033] IR (cm -1 ) v: 3240, 2980, 1740, 1690-1680, 1640, 1460-1455, 1390, 1050, 685, 670, 610.
[0034] 1 HNMR (300Hz, CDCl 3 )δ: 8.825(s, 1H), 8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com