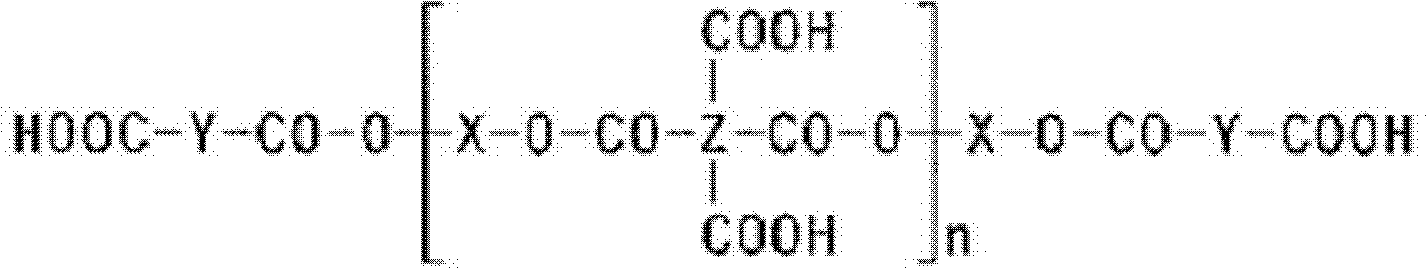Photopolymerizable resin composition
A technology of photopolymerization resin and composition, applied in optics, optomechanical equipment, instruments, etc., can solve problems such as poor uniformity of color filters, poor display quality, etc., and achieve the effect of reducing display defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
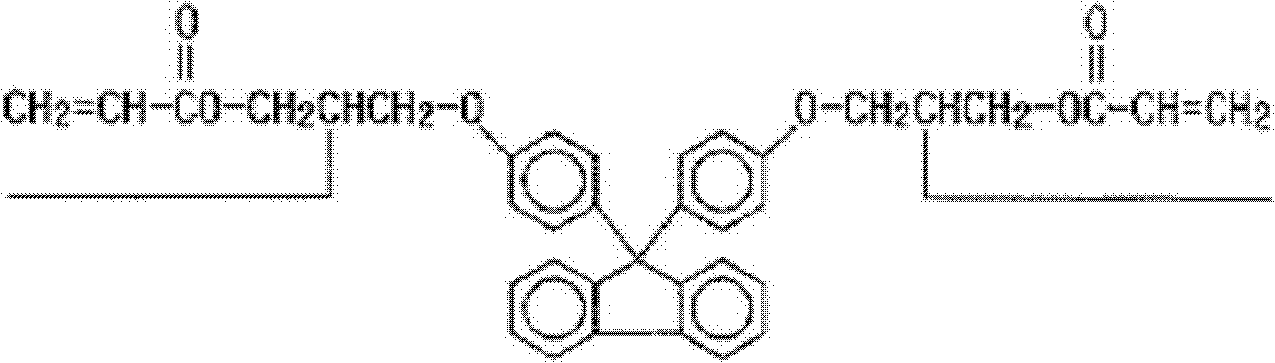
[0077] According to an exemplary embodiment of the present invention, a functional group capable of imparting hydrophobicity may be introduced into such a cardo-based compound. In particular, as described above, fluorine may be introduced.
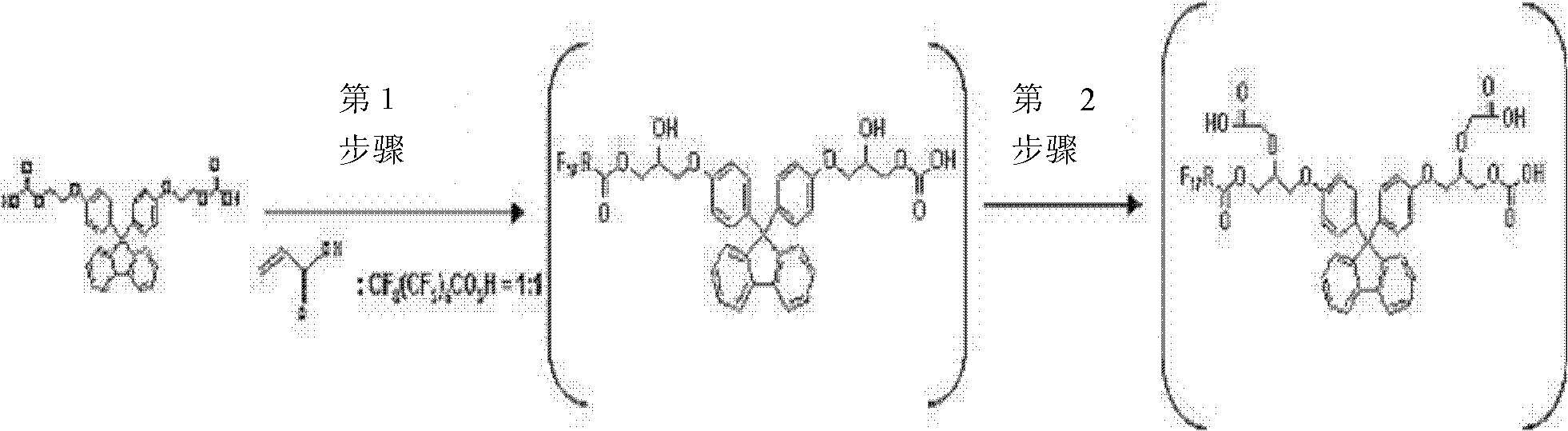
[0078] In the preparation of the cardo-based compound, introduction of fluorine and examples of compounds usable in the introduction of such a group are not limited. Specifically, it is a compound obtained by the following reaction formula 1.
[0079] Reaction 1
[0080]
[0081] Equation 1 illustrates the introduction of fluorine into a cardo-based compound and should not be construed as limiting the fluorine-containing cardo-based compounds useful in the present invention.
[0082] The cardo-based compound may be used in an amount of about 1 to 40 wt%, and preferably in an amount of about 20 to 30 wt%, based on the total solid weight of the photopolymerizable resin composition. When a fluorine-containing compound is used as the car...
preparation example 1~5
[0104] Preparation Examples 1-5: Synthesis of Alkali-Soluble Acrylic Binder Resin
[0105] The components shown in Table 1 below were charged into a 1000 ml four-necked flask, and stirred for 30 minutes while blowing nitrogen gas. Then, the temperature was gradually raised, and the reaction was carried out at 70° C. for 6 hours, then the temperature was raised to 80° C., and the reaction was carried out for another 2 hours, whereby an alkali-soluble acrylic binder resin was synthesized. In Table 1 below, units are expressed in g.
[0106] Table 1
[0107] Preparation Example 1
Preparation example 2
Preparation example 3
Preparation Example 4
Preparation Example 5
MAA
39.27
28.62
26.22
24.19
22.43
GMA
130.90
121.10
110.92
102.33
49.35
Stylized
26.18
15.41
14.12
13.02
11.21
KBM503
21.82
8.81
8.07
7.44
6.73...
preparation example 6
[0115] Preparation Example 6: Synthesis of Cardo-Based Compounds
[0116] 58g bisphenol fluorene type epoxy resin (epoxy equivalent 232), 313g PGMEA, 2.5g triethyl benzyl ammonium chloride, 0.03g hydroquinone and 18g acrylic acid join in the four-necked flask of 500ml, at 25ml / While passing nitrogen gas at a rate of min, heat to 80-90°C and dissolve. The slightly opaque solution was gradually heated and then completely dissolved at 80°C. Continue heating and stirring until the measured acid value of the solution is less than 1.0 mgKOH / g. In this regard, it takes 12 hours to achieve the desired acid number. The above solution was cooled to room temperature to obtain a colorless and transparent bisphenol fluorene type epoxy acrylate.
[0117] 300g of the bisphenol fluorene type epoxy acrylate thus obtained and 14g of 1,2,3,6-tetrahydrophthalic anhydride, 0.3g of 3,3',4,4'-biphenyltetracarboxylic dianhydride and 0.76 g of tetraethylammonium bromide was mixed, and then the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com