Liposome-encapsulated recombinant human ciliary neurotrophic factor
A ciliary neurotrophic and liposome technology, which is applied in liposome delivery, nervous system diseases, drug combination, etc., can solve the problems of clinical application impact, CNTF protein toxicity and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1rhCNTF (A 17 R 63 15) Acquisition of genes
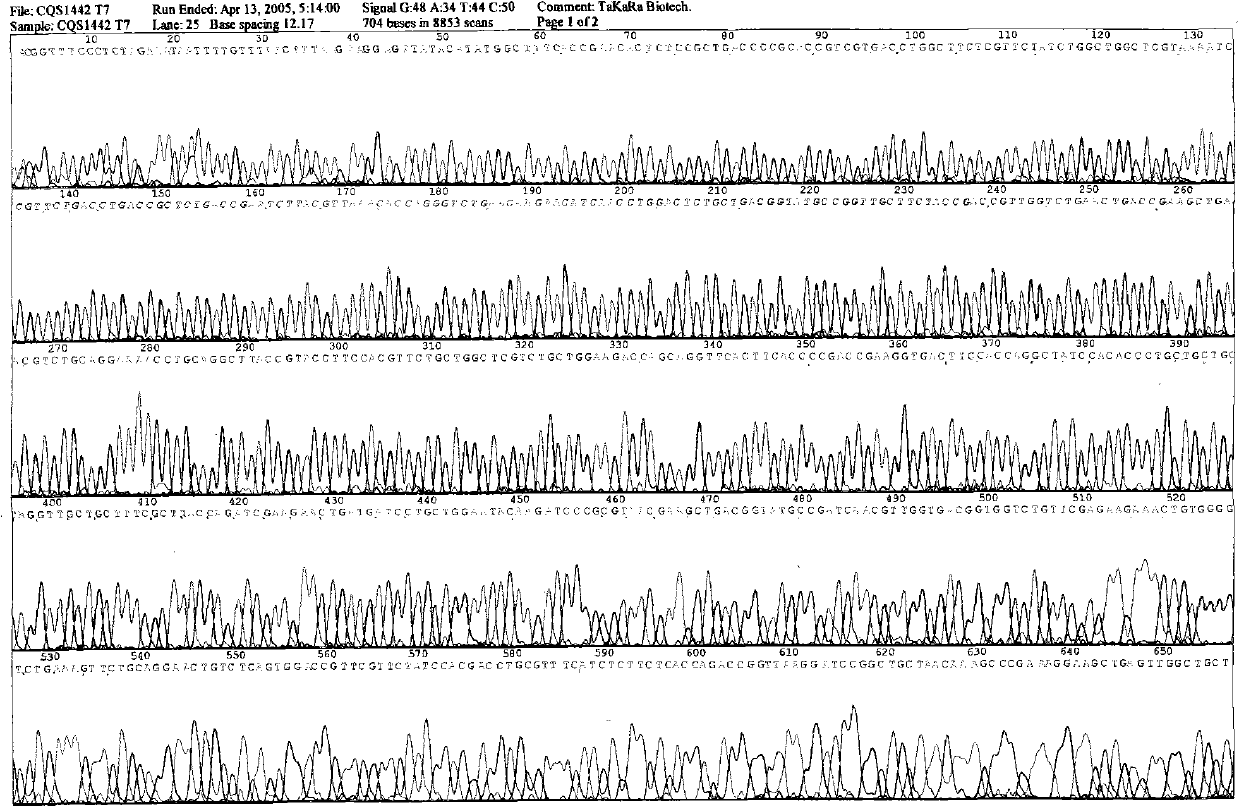
[0014] According to the CNTF cDNA sequence obtained from Gene Bank, 15 amino acids were removed from the C-terminal of the cDNA of wild-type human CNTF to increase its biological activity. At the same time, Cys at position 17 was mutated to Ala, Gln at position 63 was mutated to Arg, and a band was designed on the gene sequence. There are NdeI restriction sites and ATG translation start codons, and the downstream primers have BamHI restriction sites and TAA stop codons, all using E. coli preferred codons, the whole gene is artificially synthesized, embedded in pUC18 vector and named pUC-CNTF. Corresponding base and amino acid sequence analysis such as figure 1
Embodiment 2
[0015] Construction and expression of embodiment 2 plasmid pET-CNTF
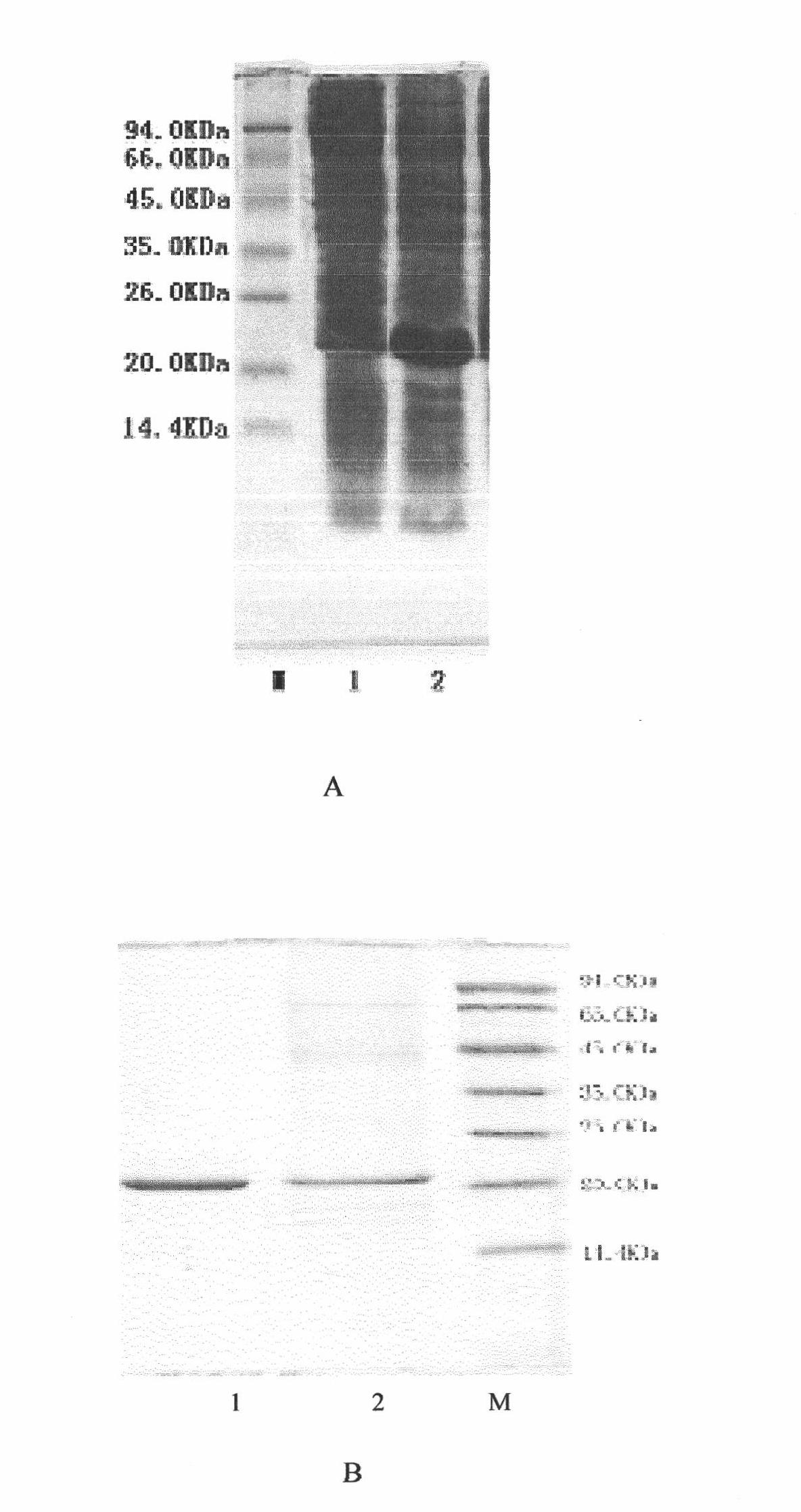
[0016] After double digestion with Nde I and BamHI, ligate with the pET-3c large fragment digested with Nde I and BamHI under the action of T4 ligase, transform Escherichia coli DH5α and coat LB-Ampicillin plates, extract positive clone plasmids, and use Nde I and BamHI double enzyme digestion identified correctly, named pET-CNTF. The recombinant expression plasmid is transformed into BL21(DE3) plyss to obtain engineering bacteria recombinantly expressing CNTF. IPTG-induced expression screened high-expressing strains and saved them. The sequencing results of engineering bacteria are as follows: figure 2 . Engineering bacteria induced expression such as image 3 a.
Embodiment 3
[0017] The separation and purification of embodiment 3 rhCNTF
[0018] Experiments proved that rhCNTF recombinant protein was expressed as inclusion body.
[0019] 1. Put the bacteria stored in the refrigerator into the bacterial lysate at a ratio of 1:10, stir at 37°C for 4 hours, and then sonicate. After cooling at 4°C, press the power P<400W for 5 seconds, with an interval of 5 seconds and sonicate 40 times, 12000r Centrifuge for 10 min at 1 / min, take the supernatant for electrophoresis, and further process the inclusion bodies.
[0020] 2. Put the inclusion body into washing solution A (20mmol / L Tris-HCl pH 8.0, 5mmol / L EDTA, 2mol / L Urea, 1% TritonX-100), ultrasonic power P<340W, working time 5 seconds, intermittent 5 seconds Ultrasound 40 times per second. After stirring for 30 minutes, centrifuge at 12,000 r / min for 15 minutes, and take 60 μL of supernatant A for SDS-PAGE.
[0021] 3. Put the inclusion body into the washing solution B (20mmol / L Tris-HCl pH 8.0, 4mol / L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com