Felodipine pharmaceutical composition based on dry process granulation
A dry granulation technology for felodipine, which is applied in the pharmaceutical field to achieve the effects of reduced production energy consumption, enhanced quality control and cost savings, and a wide range of granulation process operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
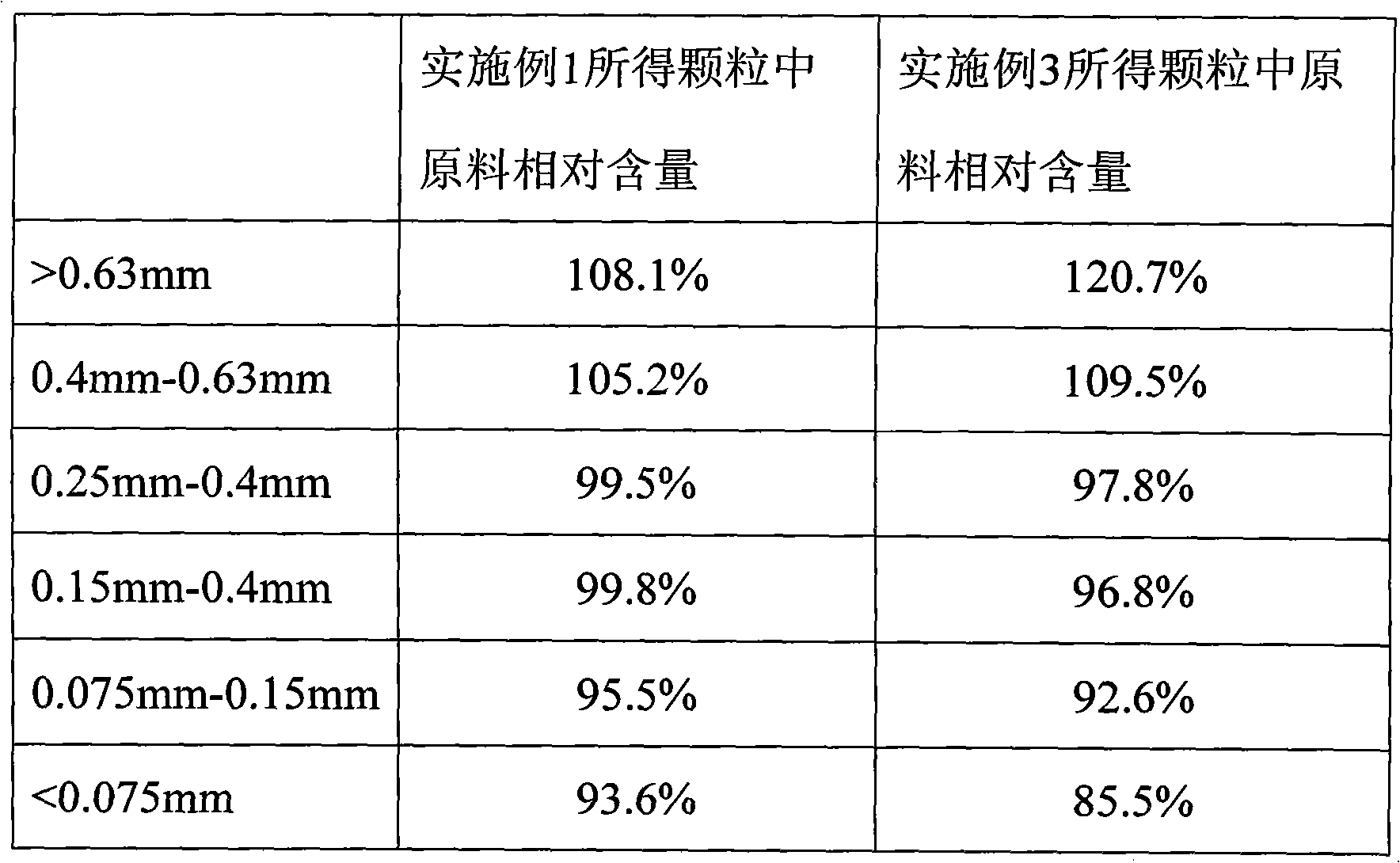
Embodiment 1
[0033] Preparation of 2.5mg Felodipine Tablets
[0034] components
[0035] Felodipine was micronized together with lactose monohydrate (premixed) and pulverized to an average particle size of 30 μm, d(0.9)=85.2 μm. Mix with other internally added materials lactose monohydrate and polyethylene glycol for 15 minutes, then add the internal lubricant glyceryl behenate and mix for 3 minutes. Dry granulation with a roller compactor. Add croscarmellose sodium and added lactose monohydrate and mix for 5 minutes, then add additional glyceryl behenate and mix for 3 minutes. Tablet to obtain the desired product.
Embodiment 2
[0037] Preparation of 2.5mg Felodipine Tablets
[0038] components
Prescription amount (mg / tablet)
percentage
Felodipine
2.5
1.25%
Lactose monohydrate (premixed)
20
10.0%
polyethylene glycol
8
4.0%
Lactose monohydrate (added)
78
39.0%
Microcrystalline Cellulose (Added)
20.3
10.15%
Glyceryl Behenate (Added)
1
0.50%
Lactose monohydrate (additional)
20
10.0%
Microcrystalline Cellulose (Extra)
40
20.0%
Croscarmellose sodium (additional)
10
5.0%
Glyceryl Behenate (additional)
0.2
0.10%
total
200
100%
[0039] Preparation Process:
[0040] Felodipine was micronized together with lactose monohydrate, and pulverized to an average particle size of 56 μm, d(0.9)=126.2 μm. Then mix with other internally added materials polyethylene glycol, microcrystalline ce...
Embodiment 3
[0041] Embodiment 3 (comparative example)
[0042] Adopt the same prescription as Example 1, but do not carry out micronization.
[0043] components
Prescription amount (mg / tablet)
percentage
Felodipine
2.5
1.25%
10
5.0%
Lactose monohydrate (added)
90
45.0%
Lactose monohydrate (additional)
52.3
26.15%
Glyceryl Behenate (Added)
1
0.5%
Microcrystalline Cellulose (Extra)
40
20.0%
Croscarmellose sodium (additional)
4
2.0%
Glyceryl Behenate (additional)
0.2
0.1%
total
200
100%
[0044] Preparation Process:
[0045] The felodipine internal addition material lactose monohydrate and polyethylene glycol were mixed for 15 minutes, and then the internal lubricant glyceryl behenate was added and mixed for 3 minutes. Dry granulation with a roller compactor. Add additional lactose monoh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com