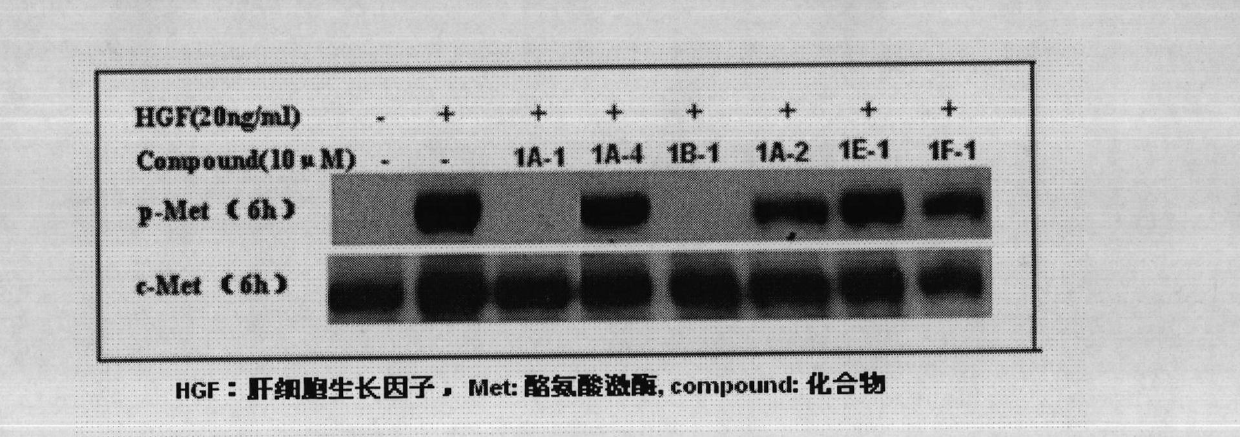
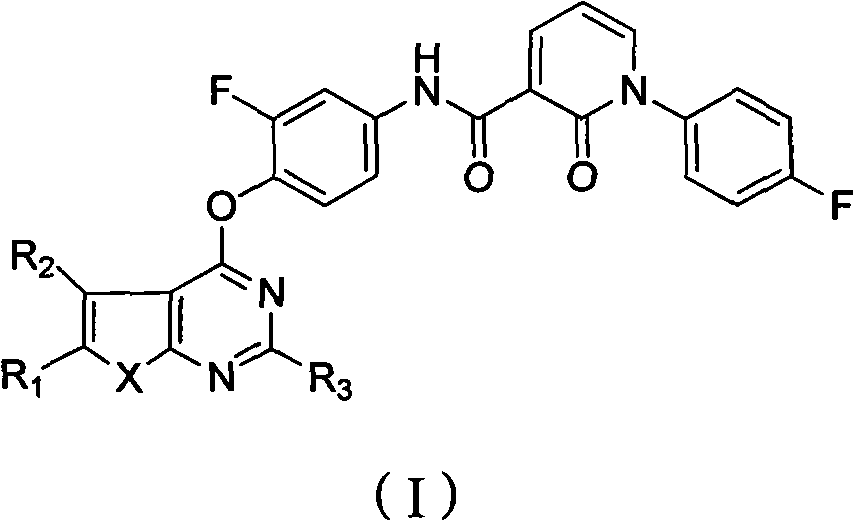
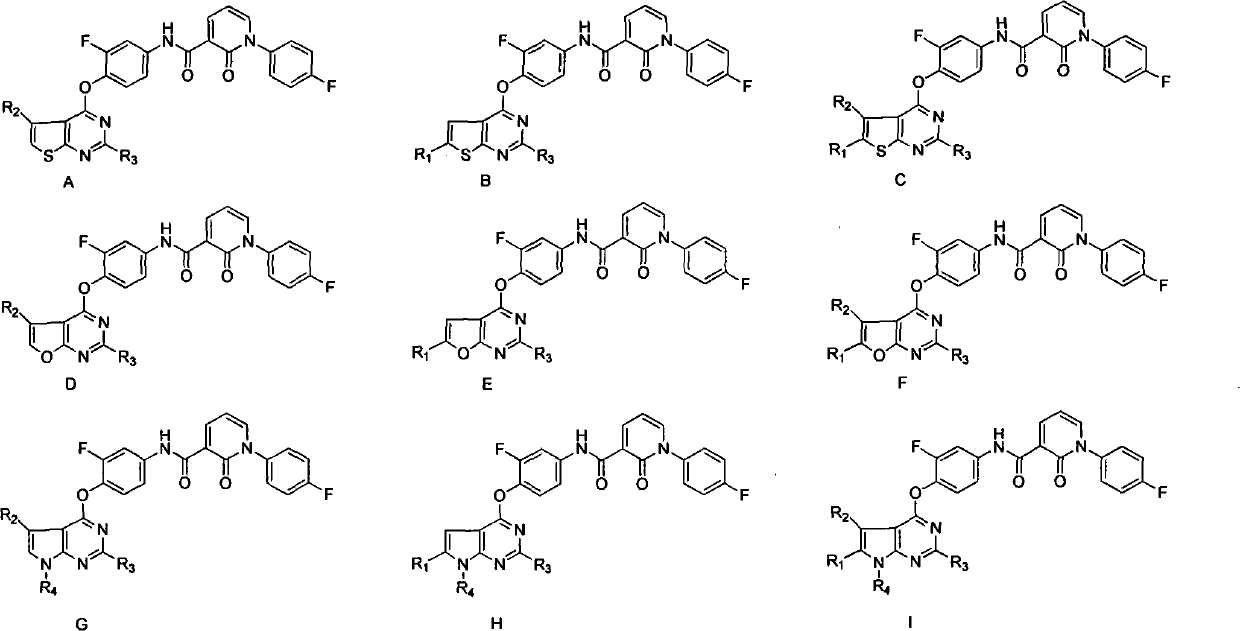
Five-membered heterocycle pyrimidine compounds, preparation method and application thereof
A technology for five-membered heterocycles and compounds is applied to a class of five-membered heterocyclic pyrimidine compounds and the fields of their preparation and use, and can solve problems such as limited structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0077] Preparation Example 1 Preparation of Compound IA-0
[0078]
[0079] 1.1 Preparation of 3-fluoro-4-(thien[2,3-d]pyrimidine-4-oxyl)aniline
[0080] Dissolve 4-amino-2-fluorophenol (80mg, 0.63mmol) in 6ml of anhydrous DMF, add NaH (25mg, 1.1mmol) under ice-cooling conditions, stir for 10 minutes, add dropwise 4-chlorothiophene[2,3 -d] Pyrimidine (65mg, 0.38mmol) in DMF (2ml), stirring in ice bath was continued for 1 hour. After the reaction was completed, add ice water dropwise to quench the reaction, extract with ether, wash the organic phase with water, wash with saturated brine, dry over anhydrous sodium sulfate, spin off the solvent, and perform column chromatography (petroleum ether: ethyl acetate = 3:1) to obtain 3-Fluoro-4-(thiophene[2,3-d]pyrimidin-4-oxyl)aniline (95 mg), yield: 95%.
[0081] 1 HNMR (300MHz, C5D5N) δ: 3.81(s, 2H), 6.52(m, 2H), 7.05(t, J=8.4Hz, 1H), 7.53(dd, J=6.0Hz, J=16.2Hz, 2H) , 8.62(s, 1H).MS-EI m / z 261(M + ).
[0082] 1.2 Preparation...
preparation Embodiment 2
[0086] Preparation Example 2 Preparation of Compound IA-1
[0087]
[0088] 2.1 Preparation of 3-fluoro-4-(5-phenylthiophene[2,3-d]pyrimidin-4-oxyl)aniline
[0089] Dissolve 4-amino-2-fluorophenol (27mg, 0.21mmol) in 3ml of anhydrous DMF, add NaH (8.2mg, 0.34mmol) under ice-cooling conditions, stir for 10 minutes, add 4-chloro-5-benzene dropwise Thiopheno[2,3-d]pyrimidine (purchased from Alfa Aesar Chemical Co., Ltd.) (30 mg, 0.12 mmol) in DMF (1 ml) was stirred in an ice bath for 1 hour. After the reaction was complete, add ice water dropwise to quench the reaction, extract with ether, wash the organic phase with water, wash with saturated brine, dry over anhydrous sodium sulfate, spin off the solvent, and separate by column chromatography (petroleum ether: ethyl acetate = 3:1) Purified to obtain 3-fluoro-4-(5-phenylthiopheno[2,3-d]pyrimidin-4-oxyl)aniline (30 mg), yield: 73%.
[0090] 1 HNMR (300MHz, CD 3 Cl) δ: 3.75(s, 2H), 6.48(m, 2H), 6.70(t, J=8.7Hz, 1H), 7.38(m, ...
preparation Embodiment 3
[0095] Preparation Example 3 Preparation of Compound IA-2
[0096] 3.1 Preparation of intermediate 4-chloro-5-(4-fluorophenyl)thiophene[2,3-d]pyrimidine
[0097]
[0098] Preparation of 2-(1-(4-fluorophenyl)ethylene)malononitrile:
[0099] Add 4-fluoroacetophenone (2.0g), malononitrile (1.9g), ammonium acetate (0.9g), and 2ml of glacial acetic acid into the reaction flask, add 20ml of m-xylene, heat to reflux to 180°C, and use a water separator The device will remove the generated water. After the reaction was completed, the solvent was evaporated to dryness, extracted with water-ethyl acetate, the organic phase was washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, the solvent was spun off, and column chromatography (petroleum ether: ethyl acetate=10:1) gave 2-(1-(4-Fluorophenyl)ethylene)malononitrile (2.2 g, 81%).
[0100] 1 HNMR (300MHz, CDCl 3 )δ: 2.62(s, 3H), 7.21(m, 2H), 7.60(m, 2H).
[0101] Preparation of 2-amino-4-(4-fluoroph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com