Preparation method of L zeolite containing gallium
A technology of zeolite and gallium source, which is applied in the field of preparation of L zeolite, can solve problems such as uneven distribution of metals and affect the application effect, and achieve the effects of superior performance, good thermal stability and hydrothermal stability, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
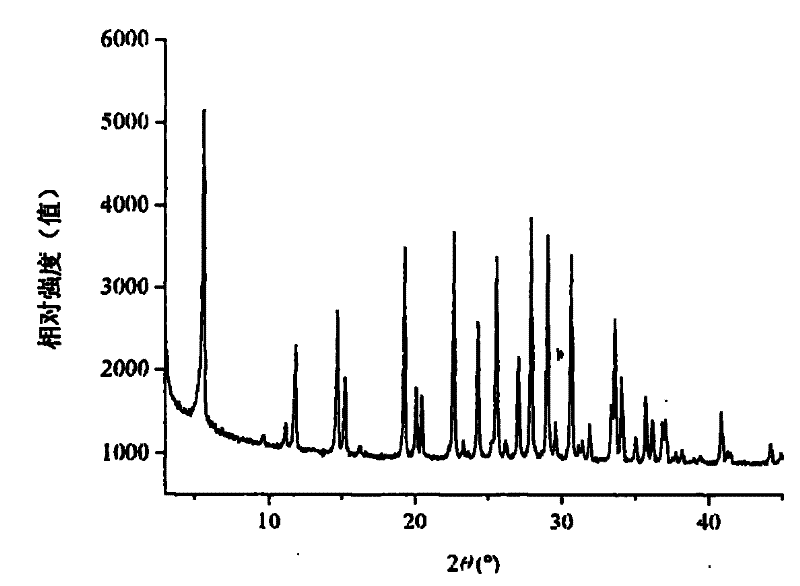
[0027] This example illustrates the preparation of Ga by using gallium metahydroxide, pseudoboehmite and silica sol 2 o 3 :Al 2 o 3 The method of zeolite L with a molar ratio of 2:8.
[0028] Get 162.9 grams of KOH, 7.0 grams of gallium metahydroxide (GaOOH), and 17.7 grams of pseudoboehmite (AlOOH) and add them to 246.0 grams of deionized water, stir and transfer to a polytetrafluoroethylene-lined autoclave for sealing. React at 150°C for 24 hours. A clear solution was obtained after cooling to room temperature, and the K in the Al / Ga precursor 2 O:Al 2 o 3 : Ga 2 o 3 :H 2 The molar ratio of O is 7:0.2:0.8:80. In this solution, slowly add 613 grams of silica sol (SiO 2 content 30wt%), stirred evenly, transferred to an autoclave, and reacted at 170°C for 36 hours. After the crystallization, the reactant was cooled to room temperature, filtered, washed and dried to obtain gallium-containing L zeolite.
Embodiment 2
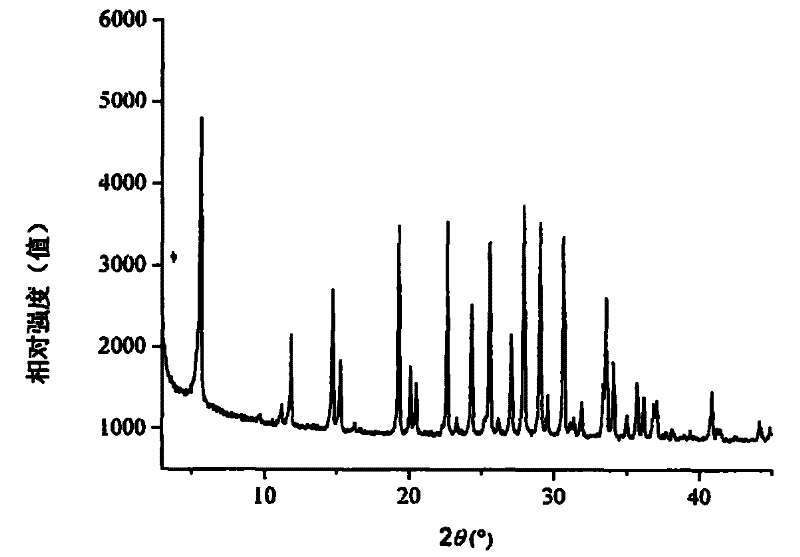
[0030] This example illustrates the preparation of Ga by using gallium metahydroxide, pseudoboehmite and silica sol 2 o 3 :Al 2 o 3 The method of zeolite L with a molar ratio of 4:6.
[0031] Take 139.6 grams of KOH, 14.0 grams of GaOOH, and 13.3 grams of AlOOH and add them into 246.0 grams of deionized water, stir evenly, transfer to a polytetrafluoroethylene-lined autoclave and seal, and react at 160°C for 18 hours. A clear solution was obtained after cooling to room temperature, and the K in the Al / Ga precursor 2 O:Al 2 o 3 : Ga 2 o 3 :H 2The molar ratio of O is 6:0.4:0.6:80. In this solution, slowly add 613 grams of silica sol (SiO 2 content 30wt%), stirred evenly, transferred to an autoclave, and reacted at 160°C for 36 hours. After the crystallization, the reactant was cooled to room temperature, filtered, washed and dried to obtain gallium-containing L zeolite.
Embodiment 3
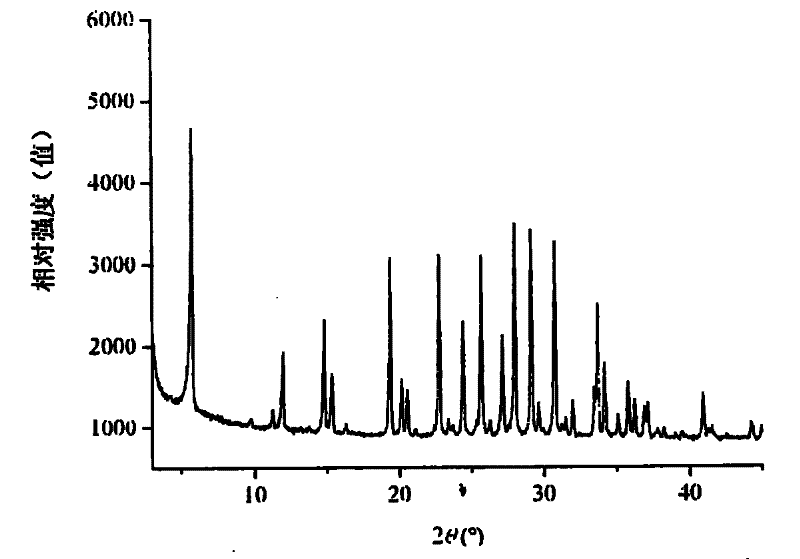
[0033] This example illustrates the preparation of Ga by using gallium metahydroxide, pseudoboehmite and silica sol 2 o 3 : Al 2 o 3 The method of zeolite L with a molar ratio of 6:4.
[0034] Take 116.3 grams of KOH, 21.0 grams of GaOOH, and 8.8 grams of AlOOH and add them to 246.0 grams of deionized water, stir evenly, transfer to a polytetrafluoroethylene-lined autoclave and seal, and react at 150°C for 36 hours. A clear solution was obtained after cooling to room temperature, and the K in the Al / Ga precursor 2 O:Al 2 o 3 : Ga 2 o 3 :H 2 The molar ratio of O is 5:0.6:0.4:80. In this solution, slowly add 613 grams of silica sol (SiO 2 content 30wt%), stirred evenly, transferred to an autoclave, and reacted at 150°C for 24 hours. After the crystallization, the reactant was cooled to room temperature, filtered, washed and dried to obtain gallium-containing L zeolite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com