Anti-vegf antibodies and their uses
An antibody and bevacizumab technology, applied in the direction of antibodies, applications, anti-inflammatory agents, etc., can solve the problems of reducing the treatment benefits of patients and limiting antibody re-administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
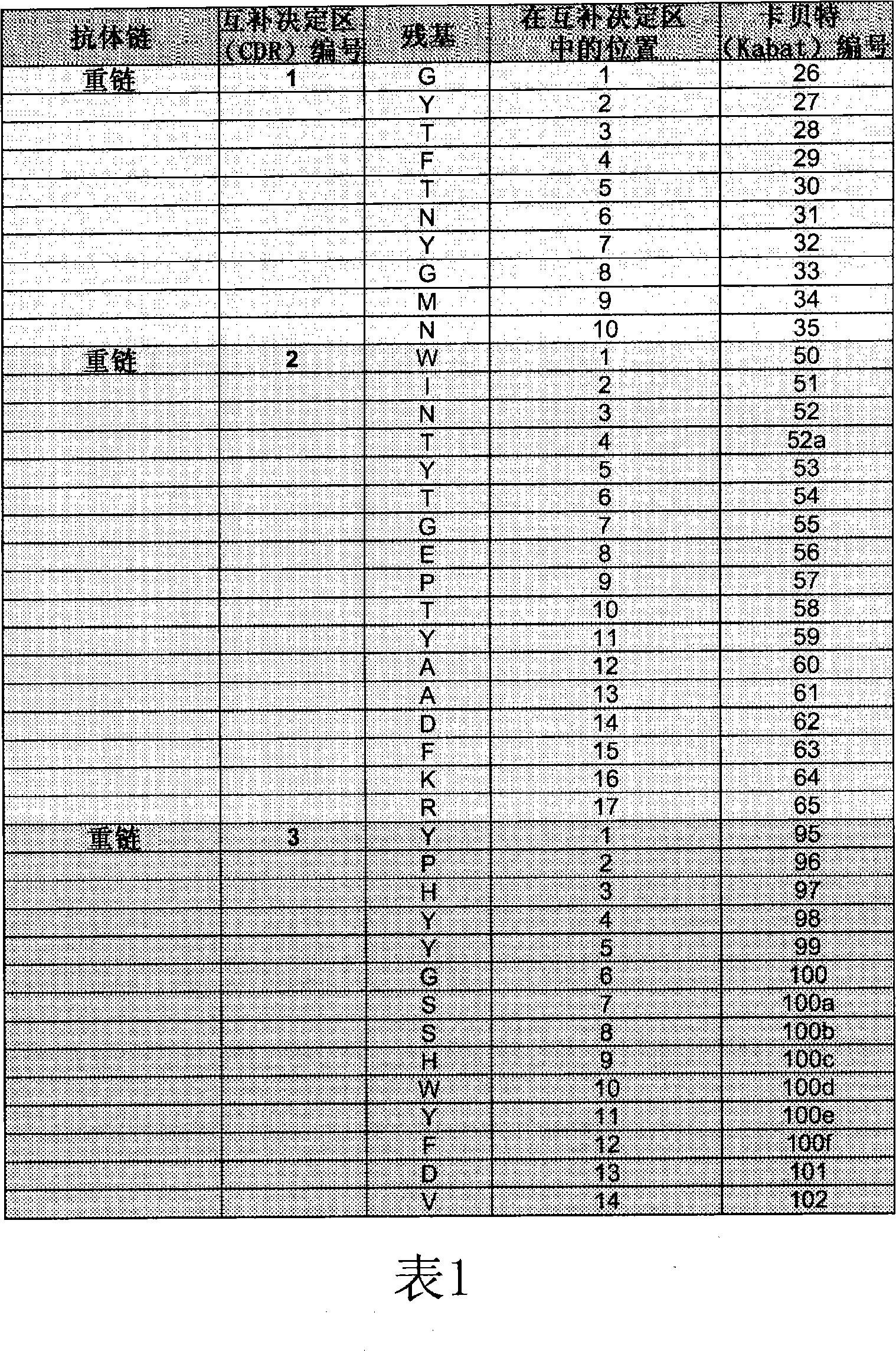
[0226] 7. Example 1: Identification of T cell epitopes of bevacizumab
[0227] 7.1 Materials and methods
[0228] 7.1.1 Peptides
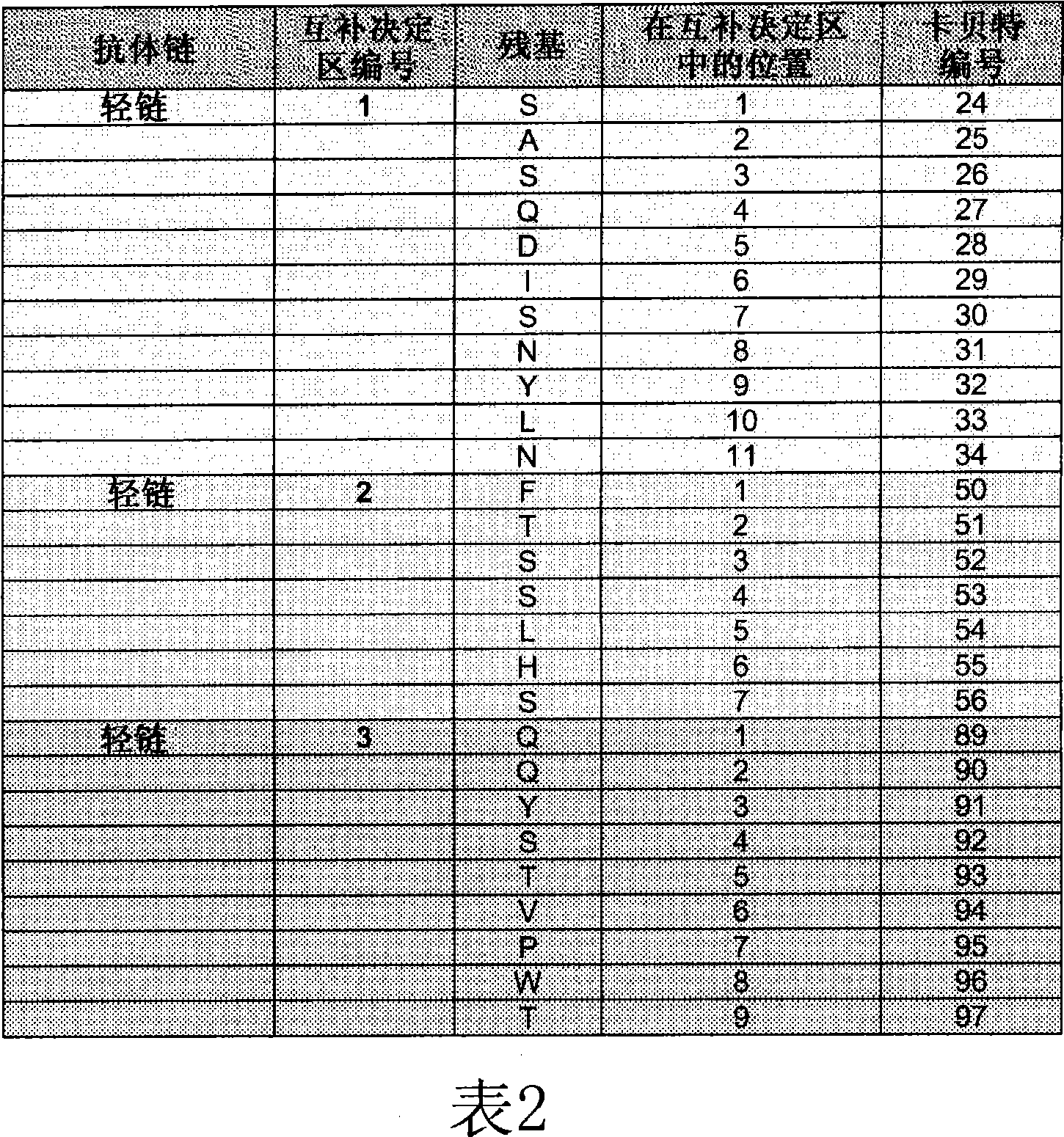
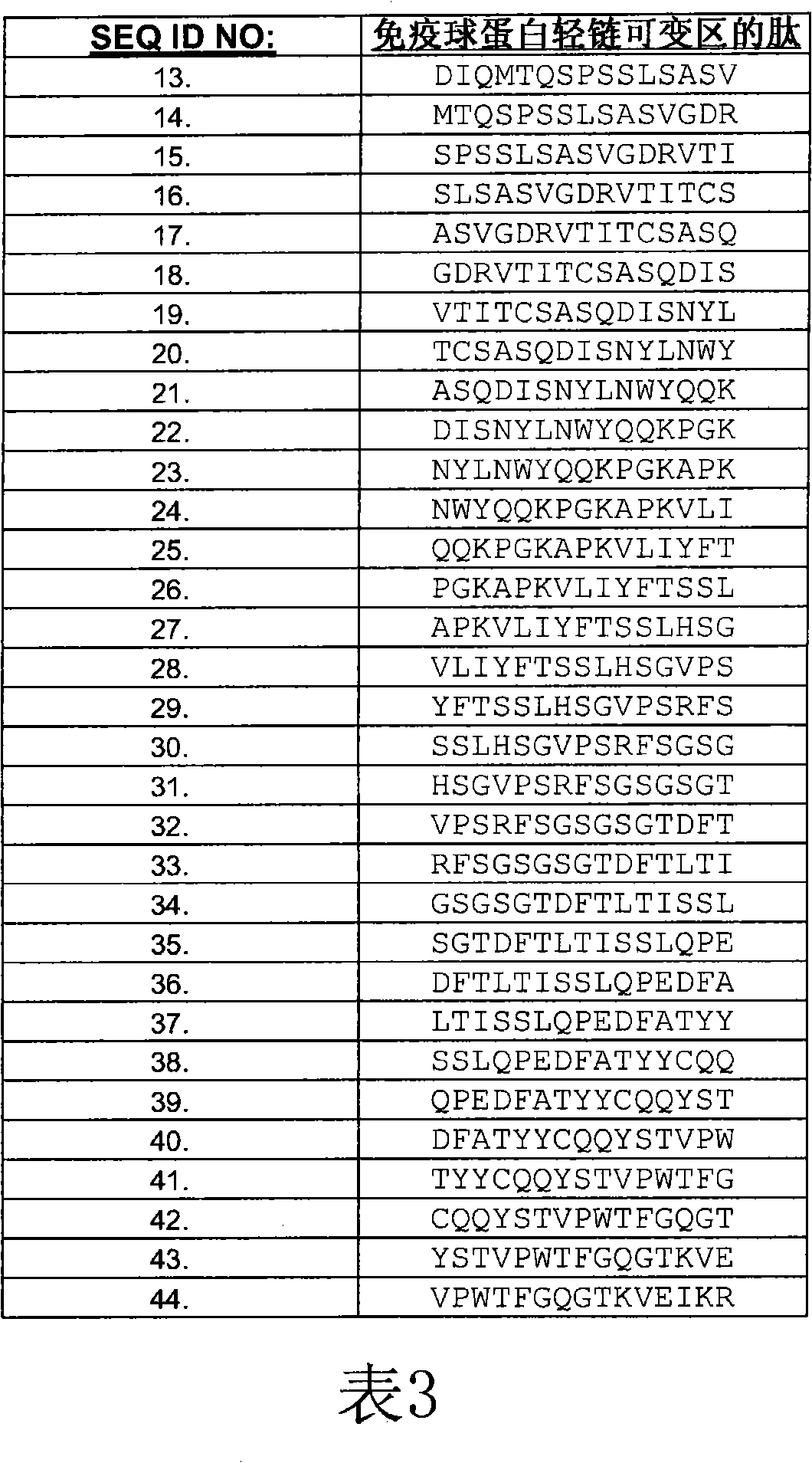
[0229] Peptides were synthesized from Mimotope (Adelaide, Australia) using a multi-pin format. The sequences of the bevacizumab light and heavy chain V regions were synthesized in the form of 15 peptides overlapping by 12 amino acids (Tables 3 and 4), for a total of 69 peptides. Peptides were lyophilized and resuspended in DMSO (Sigma-Aldrich) at approximately 1 mg / mL to 2 mg / mL. Stock peptides were stored frozen at -20°C.
[0230] 7.1.2 Human peripheral blood mononuclear cells
[0231] Community donor buffy coat product was purchased from Stanford Blood Center (Palo Alto, CA). The buffy coat material was diluted 1:1 by volume with DPBS without calcium or magnesium. Dilute the buffy coat material (25ml to 35ml) with 12.5ml of FicollPaque-PLUS (GE Healthcare) in a 50ml conical centrifuge tube (Sarsted or Coase The lower layer of the tower (...
example 2
[0245] 8. Example 2: Identification of bevacizumab variants with increased affinity for VEGF
[0246] A comprehensive mutational analysis of the bevacizumab antibody was performed to identify mutants with increased affinity for VEGF compared to bevacizumab. Candidate high-affinity mutants were analyzed by BIAcore to confirm their binding characteristics: increased affinity for VEGF compared to bevacizumab.
[0247] 8.1 Materials and methods
[0248] 8.1.1 BIAcore
[0249] Fifteen variant bevacizumab VH region constructs were cloned together with the unmodified VL region into human IgG containing 1 The plasmid was expressed in 293T / 17 cell line by transient transfection, and the antibody was purified by protein A or protein G affinity method. Antibody responses to VEGF (R&D systems, Minneapolis, Minnesota) were determined by using BIAcore 2000 and 3000 surface plasmon resonance systems (BIAcore; GE Healthcare, Piscataway, NJ). Adams (Minneapolis, MN)) affinity. Polymer w...
example 3
[0253] 9. Example 3: Selection of Deimmunized Variant Peptides
[0254] Variant peptides (Tables 14 to 16) corresponding to the immunogenic regions of bevacizumab (see Example 1) were generated. Variant peptides were selected according to the comprehensive mutational analysis described in Example 2, in which CDR modifications were identified that did not substantially reduce the binding affinity of bevacizumab for VEGF.
[0255] A total of 77 peptides were synthesized and tested based on antigen binding studies, including two syntheses of each parental 15 peptides. A total of 93 donors were tested with the parental and variant peptides using the method described in Section 7.1 and the results are shown in Figure 4A to Figure 4C middle. Specifically, Figure 4A to Figure 4C Demonstration of CD4+ T cell responses to mutant bevacizumab epitope peptides. The average response to the unmodified parental epitope sequence is indicated by open markers. Large circles indicate se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com