Process method for synthesizing 1, 2-benzisothiazdin-3-ketone
A technology of benzisothiazoline and process method, applied in the direction of organic chemistry and the like, can solve the problems of a large amount of waste gas, high cost, long process route, etc., and achieve the effects of simplifying synthesis steps, reducing costs, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
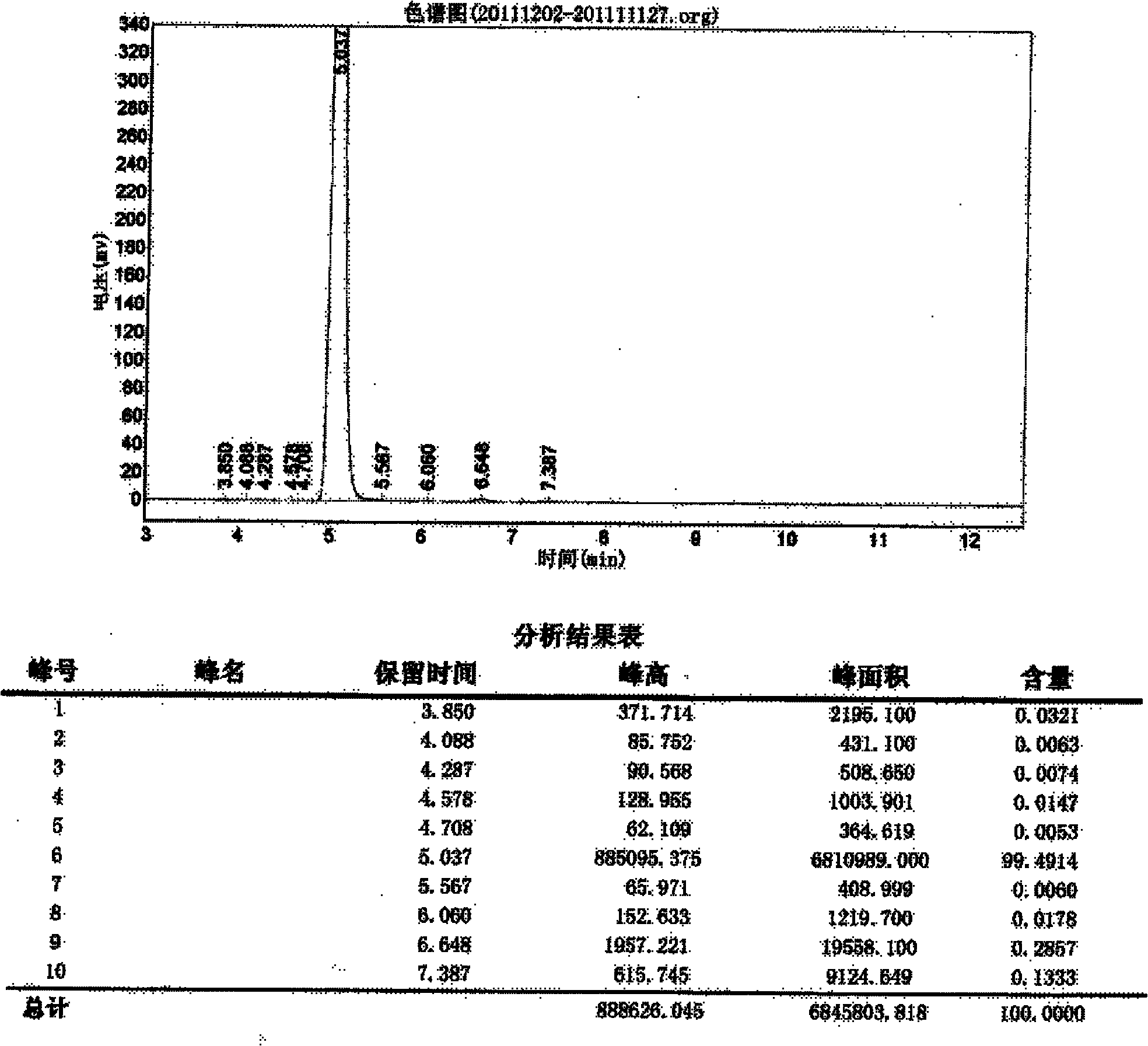
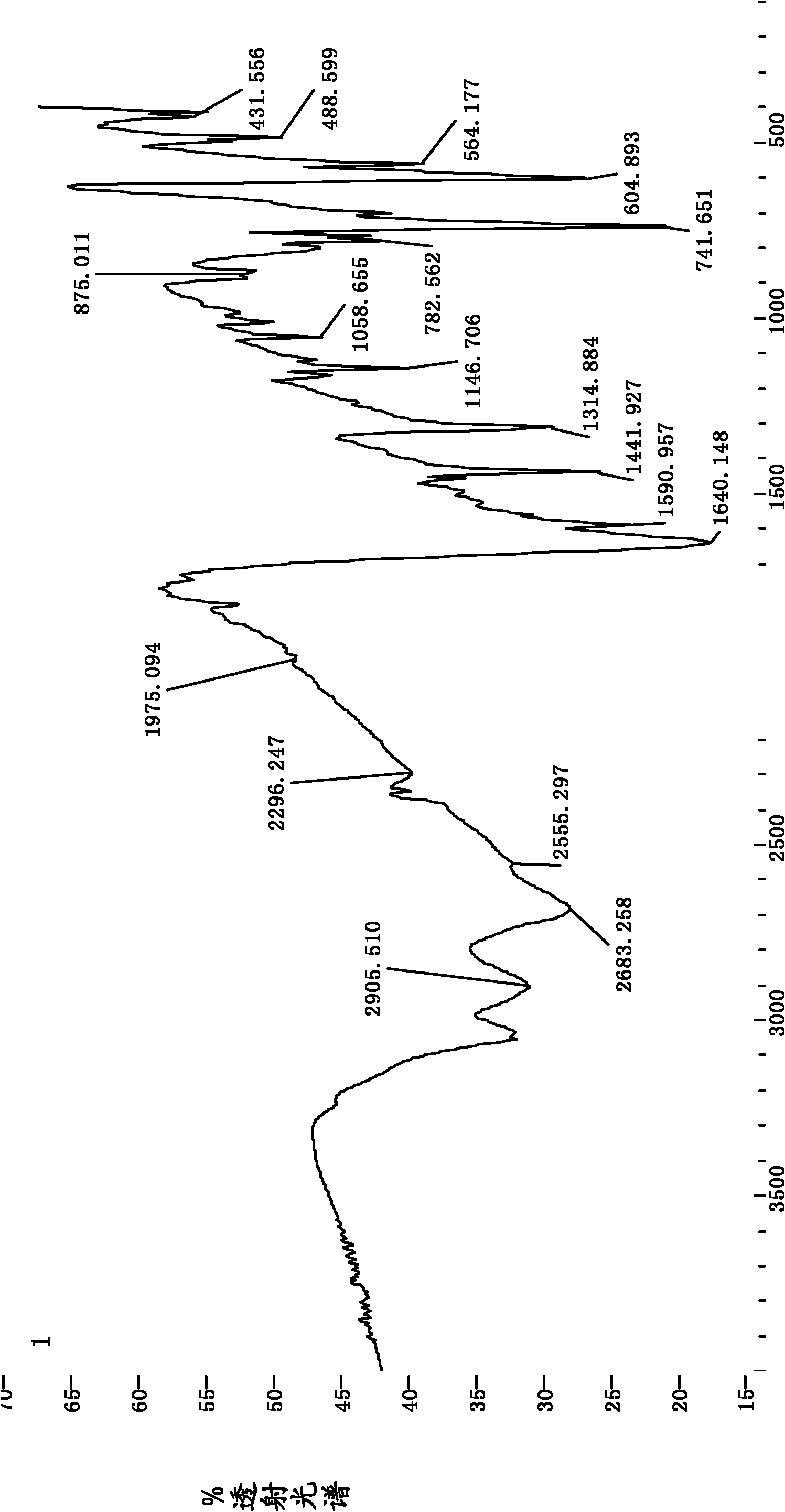
Examples
Embodiment 1
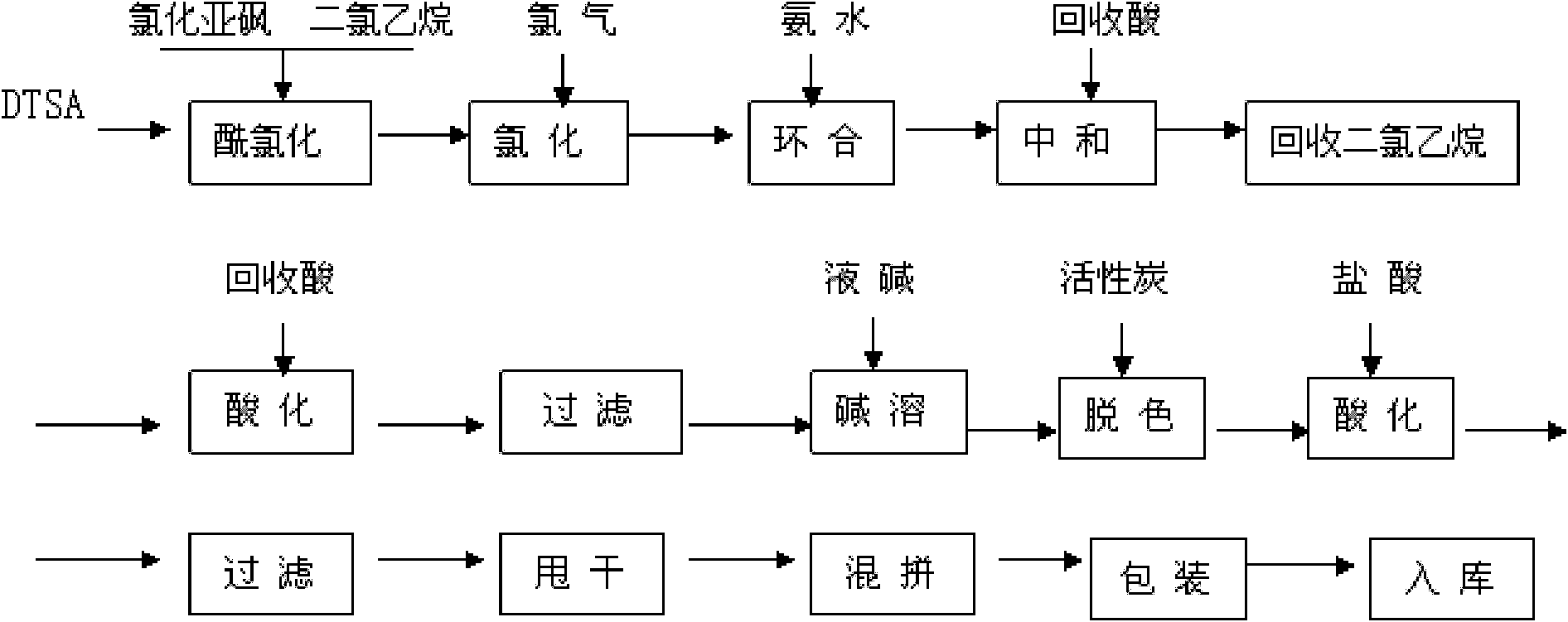
[0036] Add 46.7 grams of o-chlorobenzonitrile, 30 grams of anhydrous sodium sulfide and 550 ml of DMF under nitrogen protection in a 1000 ml four-necked bottle, and react at 90 ° C for 3 hours. After the reaction is completed, cool down and distill the solvent DMF under reduced pressure. Add 500ml of water to the compound, filter with suction, place the filtrate in an ice-water bath, and acidify it to PH=2 with 10% hydrochloric acid, a yellow oily liquid appears in the lower layer, extract it with ethyl acetate, combine the organic matter, evaporate the solvent ethyl acetate, and obtain the product yellow liquid 32 gram.
[0037] Put 200 grams of o-mercaptobenzonitrile, 680 grams of chlorinated benzene, and 72 grams of water into a 1000 mL four-necked bottle, start stirring, cool down, control the temperature at 10°C-20°C, and pass chlorine gas, about 100 grams, and sample for liquid phase analysis When the peak of the raw material is 1%, continue to pass the chlorine until th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com