Saturated acid hydrolysis technology of alkylchlorosilane
A technology of alkyl chlorosilane and acid hydrolysis, applied in the field of alkyl chlorosilane saturated acid hydrolysis process, can solve the problems of affecting the purity of hydrogen chloride gas, supersaturation of concentrated acid, low mixing efficiency, etc., and achieves regular molecular shape and molecular weight distribution, Low chlorine content and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0033] In order to make the technical means, creative features, objectives and effects of the present invention easy to understand, the following will further illustrate the present invention in combination with practical examples, but the embodiments of the present invention are not limited thereto.
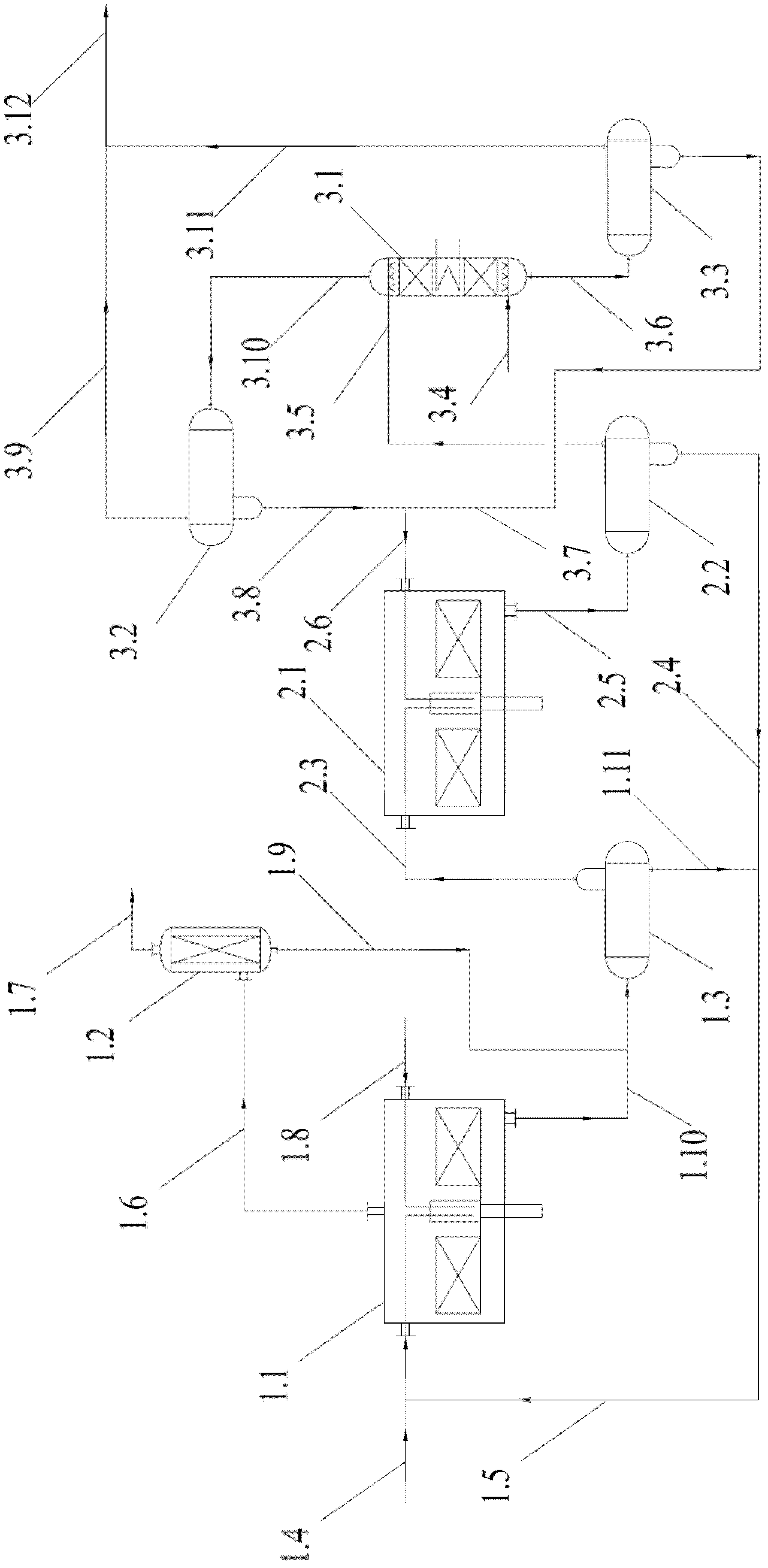
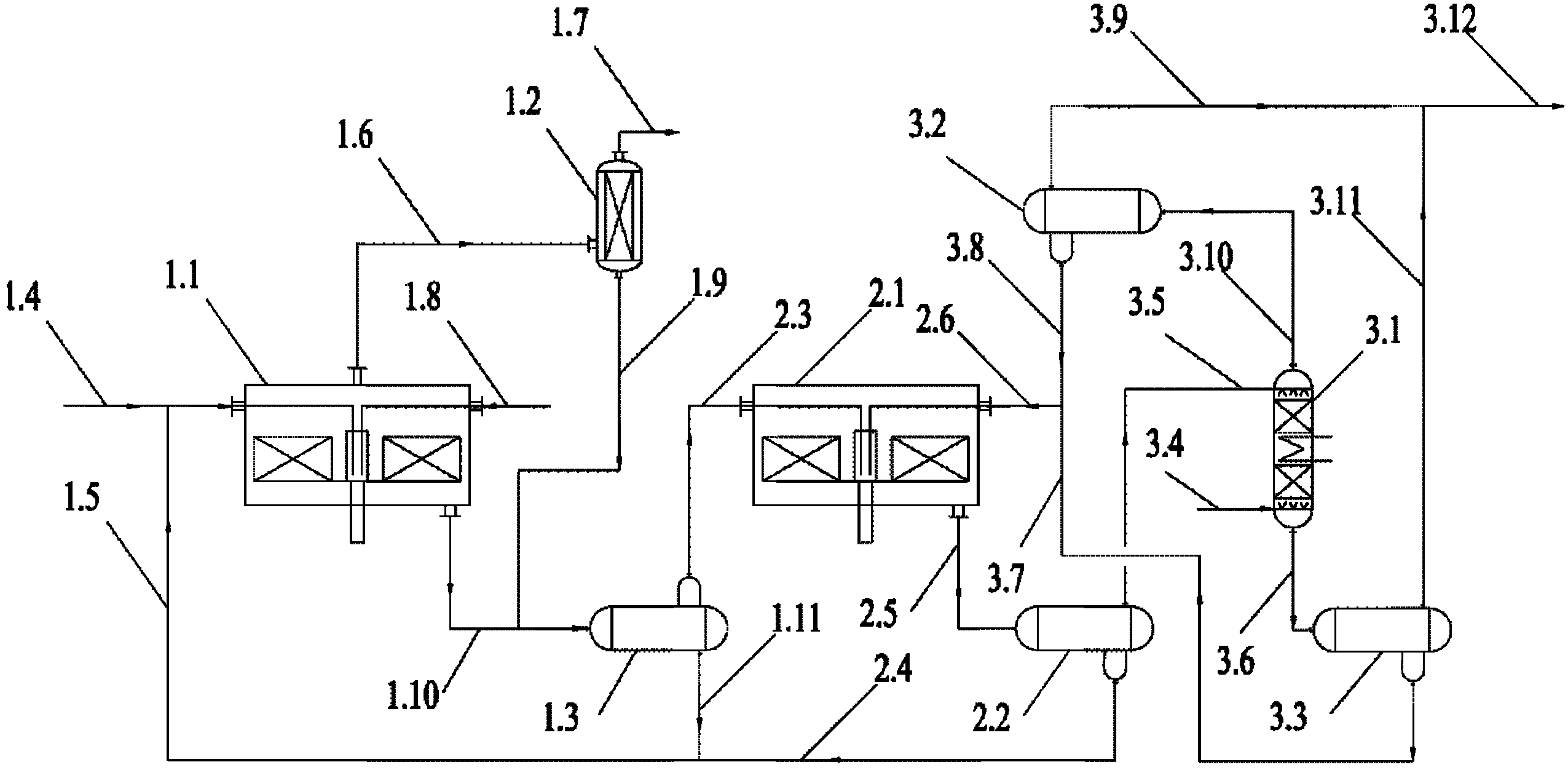
[0034] like figure 1As shown, the first step is a saturated acid hydrolysis reaction. The alkyl chlorosilane 1.8 and the saturated acid 1.5 enter the saturated acid hydrolysis strengthening reactor 1.1 for hydrolysis. The reaction temperature is controlled at 45° C. and the operating pressure is 0.3 MPa. The reaction produces liquid saturated acid hydrolyzate 1.10 and gaseous hydrogen chloride 1.6. The hydrogen chloride gas 1.6 generated by saturated acid hydrolysis can be returned to the chlorosilane synthesis section after being purified by the demister 1.2. The saturated acid hydrolysis product 1.10 passes through the saturated acid hydrolysis phase separator 1.3, and the se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com