Application of porcine pancreas lipase as catalyst for Michael addition reaction
A porcine pancreatic lipase and addition reaction technology, which is applied in the application field of porcine pancreatic lipase as a catalyst for Michael addition reaction, can solve the problems that benzalone coumarin has not been reported at home and abroad, etc., and achieve excellent catalyst and good catalytic activity , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Confirmation of the activity of porcine pancreatic lipase to catalyze the Michael addition reaction of 4-hydroxycoumarin and α,β-unsaturated ketone
[0025] In this example, the reaction of 4-hydroxycoumarin and benzylidene acetone (that is, 4-phenyl-3-buten-2-one) was used as a template reaction to catalyze 4-hydroxycoumarin and α , The activity of the Michael addition reaction of β-unsaturated ketones was confirmed. The experimental method is: add 4-hydroxycoumarin (0.5mmol) and benzylidene acetone (0.6mmol) into the reaction flask, add no catalyst or add catalyst (50mg), then add 0.1ml water and 0.9ml DMSO, and control the temperature Stir and react at 25°C for 144 hours; after the reaction, remove the enzyme by filtration, dilute the filtrate with 25ml of water and extract with ethyl acetate, combine the dichloromethane extracts, dry with anhydrous sodium sulfate, filter, and distill the filtrate to remove the solvent under reduced pressure, then Purif...
Embodiment 2
[0030] Example 2. Methodological study on the Michael addition reaction of 4-hydroxycoumarin and α,β-unsaturated ketone catalyzed by porcine pancreatic lipase
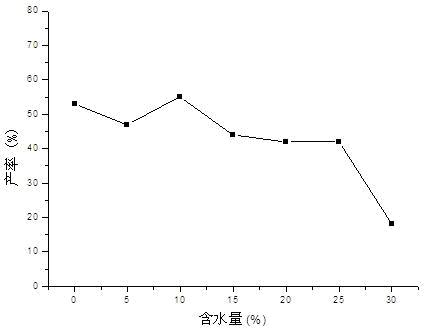
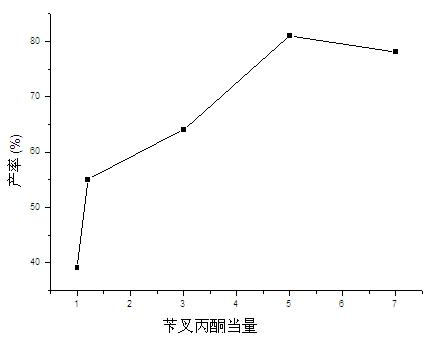
[0031] In this embodiment, the reaction of 4-hydroxycoumarin and benzylidene acetone is used as a template reaction, and the main influencing factors of the Michael addition reaction of porcine pancreatic lipase catalyzed 4-hydroxycoumarin and α, β-unsaturated ketones ( Solvent, water content, substrate feed ratio) were systematically studied.
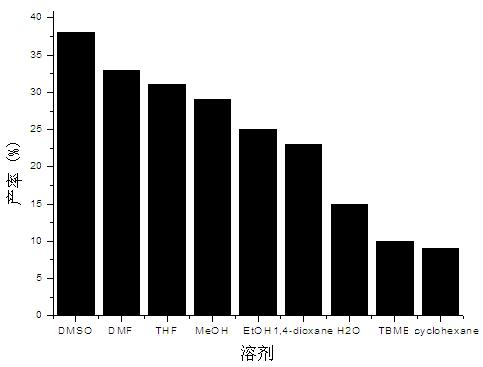
[0032] 1. The influence of solvent
[0033] Add 4-hydroxycoumarin (0.5mmol), benzylidene acetone (0.6mmol) and porcine pancreatic lipase (50mg) into the reaction flask, then add 0.1ml of water and 0.9ml of organic solvent, and stir the reaction at 25°C for 96 Hour; Aftertreatment method is the same as embodiment 1. The result is as figure 1 As shown, porcine pancreatic lipase showed the best catalytic activity in DMSO, and the highest yield reached 38%; it showed better ca...
Embodiment 3
[0038] Example 3. Porcine pancreatic lipase catalyzes the Michael addition reaction of 4-hydroxycoumarin and α,β-unsaturated ketone to synthesize benzylacetone coumarin and its derivatives
[0039] This example investigates the versatility of porcine pancreatic lipase-catalyzed Michael addition reaction of 4-hydroxycoumarin and α,β-unsaturated ketone to synthesize warfarin and its derivatives. The experimental method is: add 4-hydroxycoumarin (0.5mmol), α,β-unsaturated ketone (2.5mmol) and porcine pancreatic lipase (50mg) into the reaction bottle, then add 0.1ml water and 0.9ml DMSO, The reaction was carried out under temperature control and stirring for 144 hours, and the aftertreatment method was the same as in Example 1. The results are shown in Table 2.
[0040] Table 2 Michael addition reaction of 4-hydroxycoumarin and α,β-unsaturated ketone catalyzed by porcine pancreatic lipase
[0041]
[0042]
[0043] It can be seen from Table 2 that (1) porcine pancreatic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com