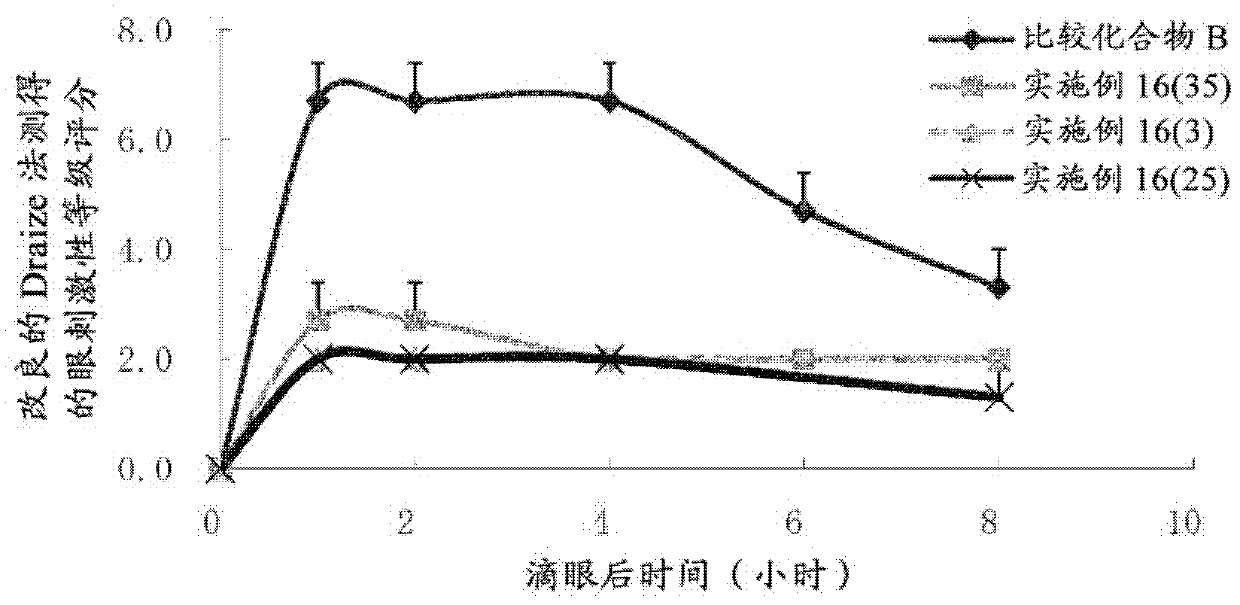
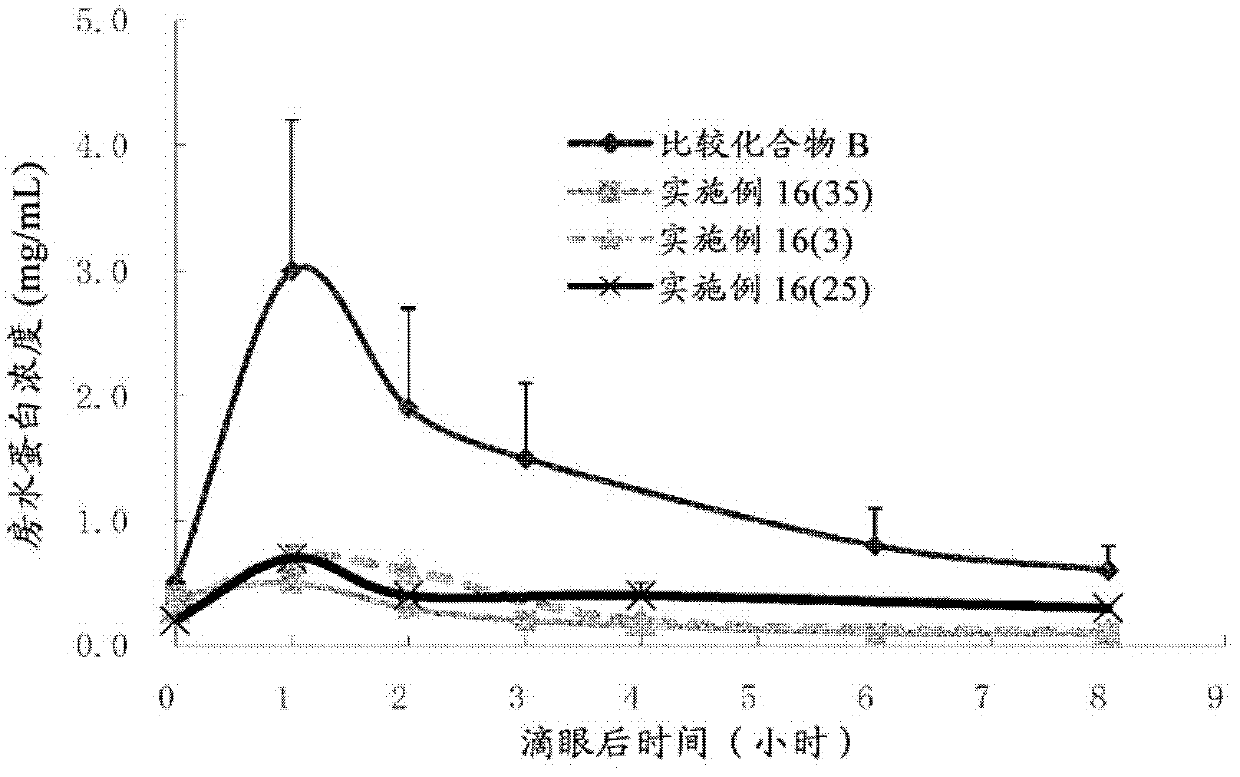
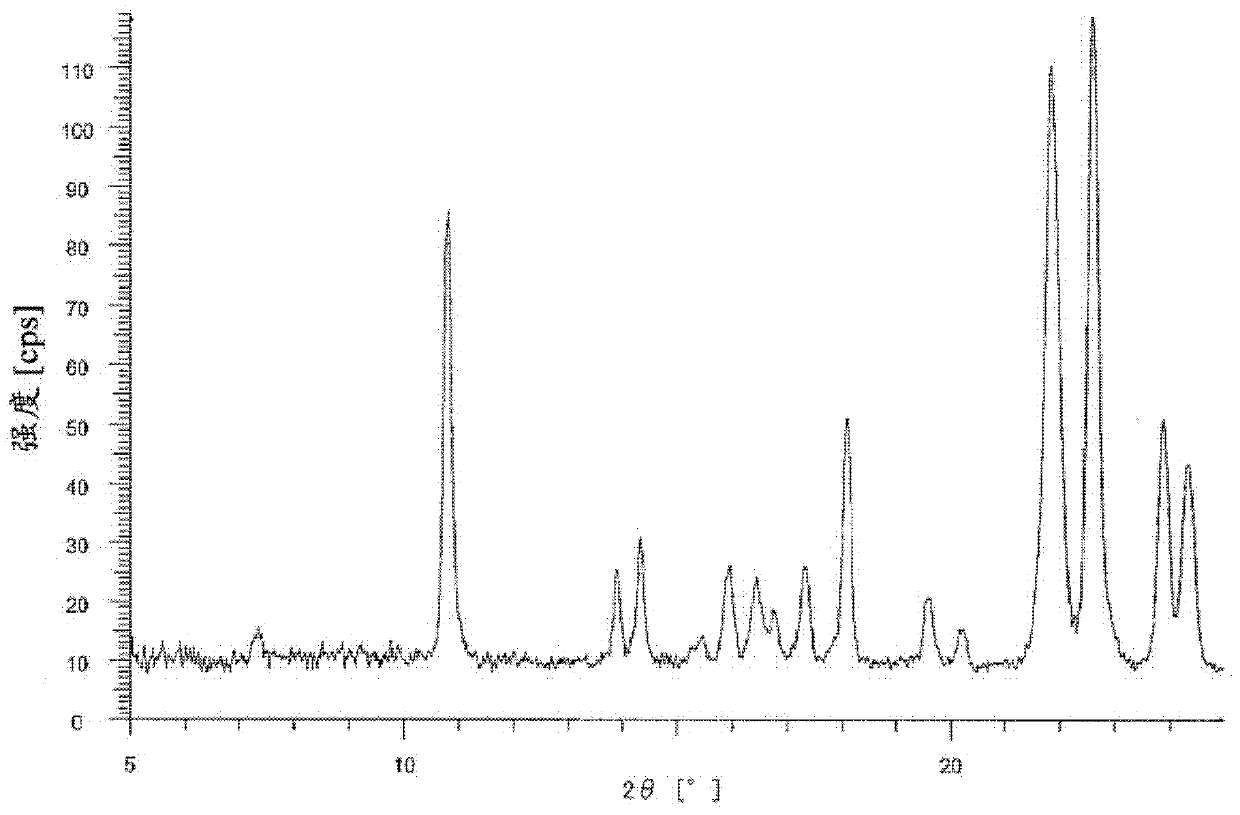
Bicyclic compound and use thereof for medical purposes
A compound and solvate technology, applied in the field of bicyclic compounds and their medical applications, can solve the problems of low stimulant effect, no record or teaching, etc., and achieve a strong effect of lowering intraocular pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0188] [Preparation method of the compound of the present invention]
[0189] The compounds of the invention can be prepared by known methods, for example by Comprehensive Organic Transformations: A Guide to Functional Group Preparations 2 nd Edition (Richard C. Larock, John Wiley & Sons Inc, 1999), or can be prepared by appropriately modifying the methods disclosed in the examples and using in combination.
[0190] Among the compounds represented by the general formula (I), for the compounds represented by the general formula (I-2),
[0191] [chemical formula 26]
[0192]
[0193] As shown below, the α chain represents the β configuration, and the R 1 on behalf of COOR 2 , R 5 represents a hydroxyl group, and R 6 and R 7 One represents hydrogen, the other represents hydroxyl,
[0194] That is the compound shown in general formula (I-2-a):
[0195] [chemical formula 27]
[0196]
[0197] (wherein all symbols represent the same meaning as above)
[0198] Can us...
Embodiment 1
[0295] Example 1: (3aR, 4S, 5R, 6aS)-4-({[dimethyl(2-methyl-2-propyl)silyl]oxy}methyl)-5-(tetrahydro- 2H-pyran-2-yloxy)hexahydro-2H-cyclopenta[b]furan-2-one
[0296] Under ice cooling, to (3aR, 4S, 5R, 6aS)-4-(hydroxymethyl)-5-(tetrahydro-2H-pyran-2-yloxy)hexahydro-2H-cyclopentadiene [b] Imidazole (29.22g) and tert-butyldimethylsilyl chloride (30.87g) were successively added to a solution of furan-2-one (50g) in dimethylformamide (hereinafter sometimes abbreviated as DMF) (100mL) , and the mixture was stirred at room temperature for 3.5 hours. After the reaction was completed, a small amount of ethanol was added, the reaction solution was poured into ice water, and extracted with ethyl acetate:hexane (2:3). The extract was washed with 1N hydrochloric acid, saturated aqueous sodium bicarbonate, and saturated brine, dried over sodium sulfate, and concentrated under reduced pressure to obtain the title compound (76.2 g) having the following physical properties:
[0297] TLC: R...
Embodiment 2
[0298] Example 2: (3aR, 4S, 5R, 6aS)-4-({[dimethyl(2-methyl-2-propyl)silyl]oxy}methyl)-5-(tetrahydro- 2H-pyran-2-yloxy)hexahydro-2H-cyclopenta[b]furan-2-ol
[0299] Under an argon atmosphere, the anhydrous toluene (390 mL) solution of the compound (76.2 g) prepared in Example 1 was cooled to -70° C., and a toluene solution of 1 mol / L diisobutylaluminum hydride ( 212.4 mL), which was then stirred for 30 minutes. After completion of the reaction, the reaction solution was diluted with tert-butyl methyl ether (hereinafter sometimes abbreviated as MTBE) (400 mL), and a saturated aqueous sodium sulfate solution was added. The precipitated white precipitate was filtered using Celite (trade name), and the solvent was concentrated under reduced pressure to obtain the title compound (80.7 g) having the following physical properties:
[0300] TLC: Rf 0.30 (hexane:ethyl acetate=3:1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com