Continuous synthesis method for acetylacetone metallic compound
A metal compound, acetylacetone technology, applied in the field of continuous synthesis of acetylacetonate metal compounds, can solve the problems of high equipment requirements, complicated operation process, low product purity, etc., and achieves wide application prospect, simple operation process, and product yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
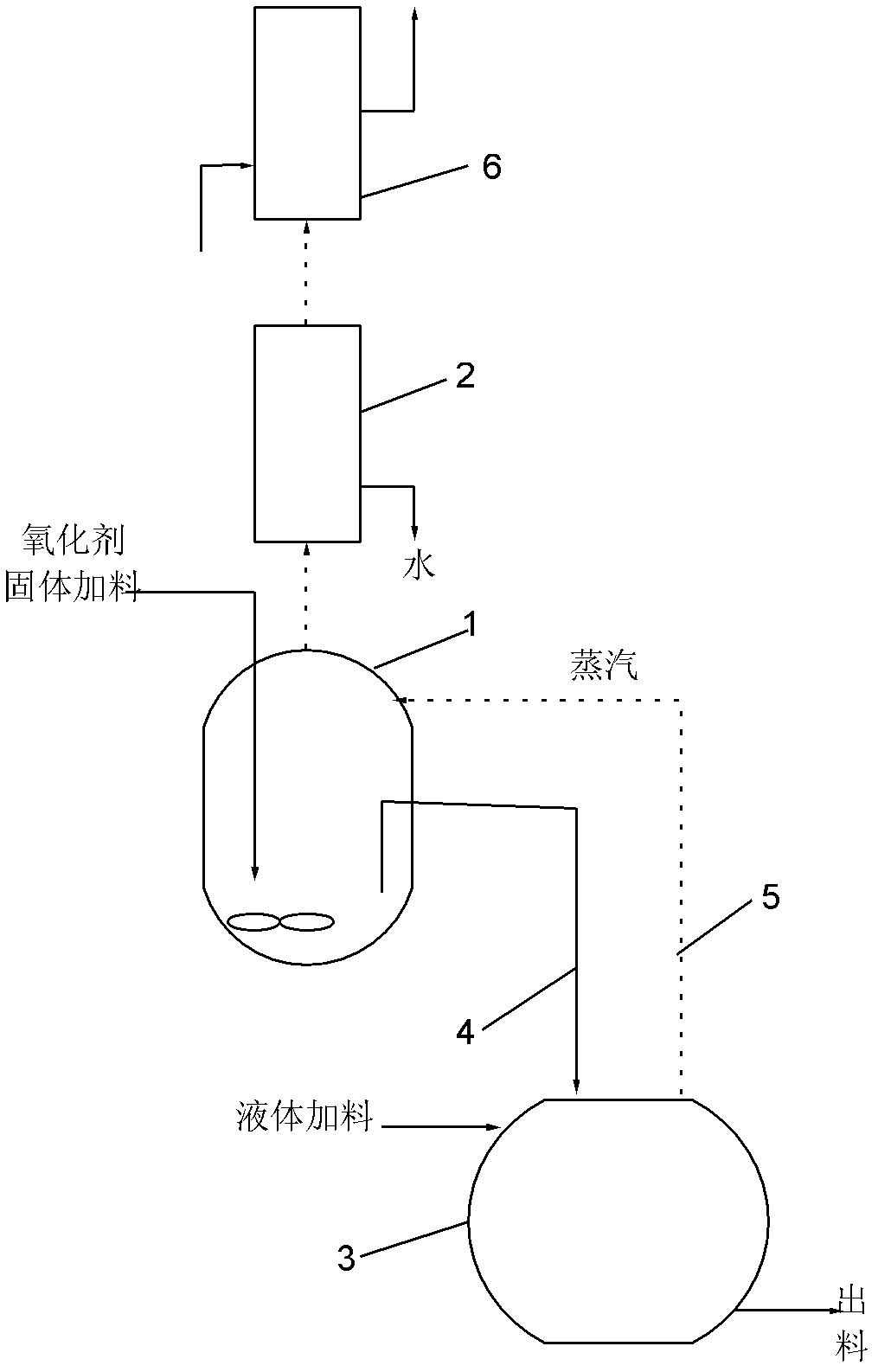
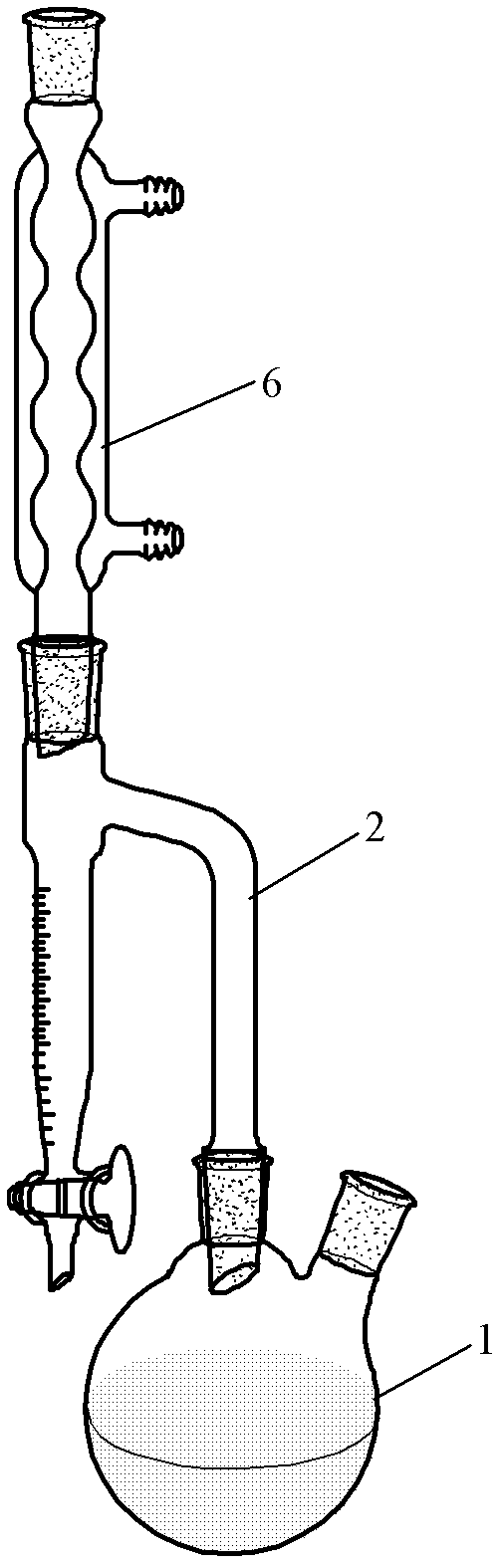
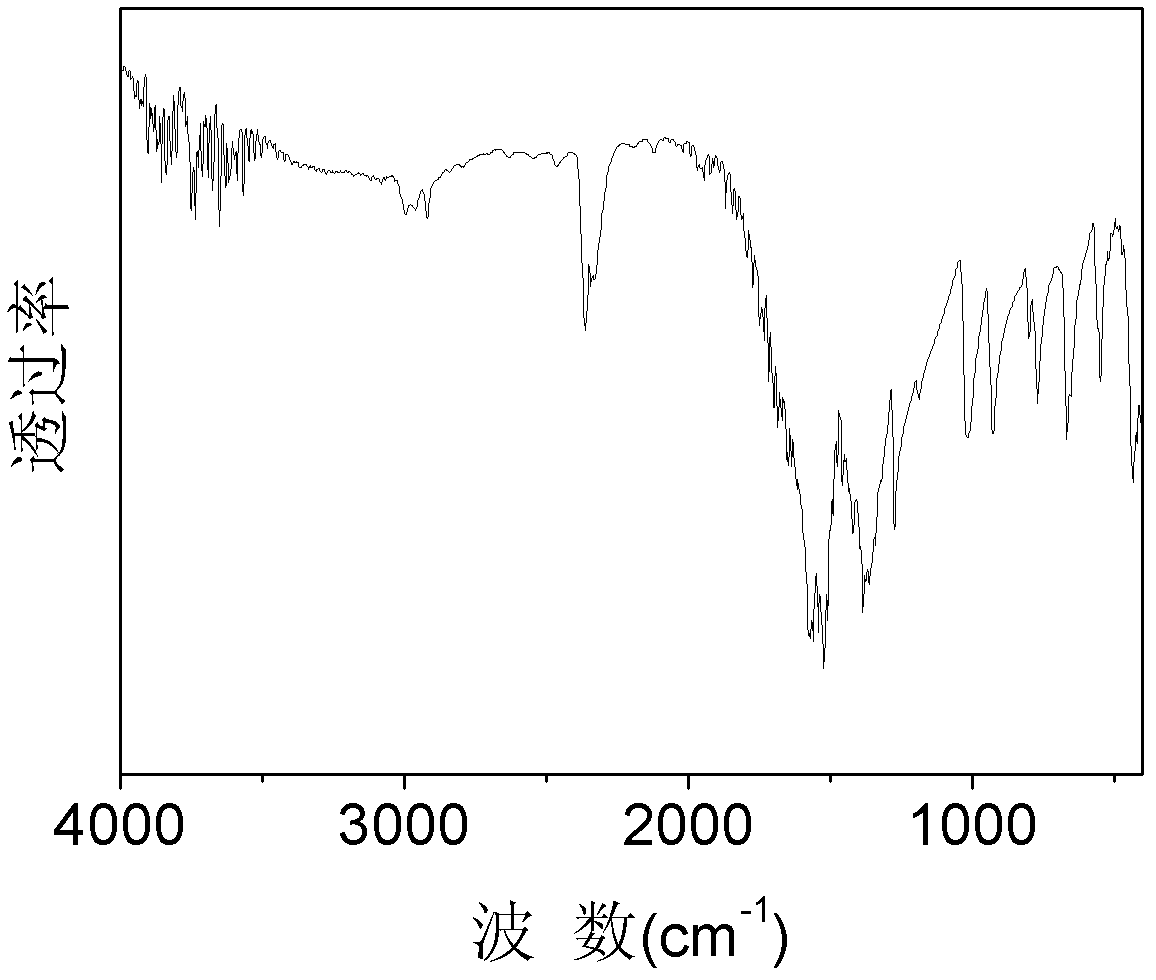
[0057] Add 8 g Fe to Reactor 1 2 o 3 , 20mL of xylene and 50mL of acetylacetone, add 50mL of acetylacetone to the product collector 3, start magnetic stirring, first heat the product collector 3 until the acetylacetone starts to reflux, then start heating the reactor 1 to reflux, stop after 2 hours of reaction Heated, and after the product collector 3 was cooled, it was placed in an ice bath, reddish-brown crystals were precipitated, filtered with suction, and reddish-brown iron acetylacetonate was obtained after precipitation and drying, with a yield of 90%. The purity of the obtained iron acetylacetonate was determined to be 98.5% by chelate titration. The infrared spectrogram of gained iron acetylacetonate is as image 3 shown.
Embodiment 2
[0059] 5 g Fe was added to batch reactor 1 2 o 3 , 50mL carbon tetrachloride and 50mL acetylacetone, start magnetic stirring, heat the reactor 1 to reflux, stop heating after 12 hours of reaction, filter while it is hot, put the filtrate in an ice bath, precipitate reddish-brown crystals, suction filter, precipitate After drying, reddish-brown iron acetylacetonate was obtained with a yield of 85%. The X-ray diffraction spectrogram of gained iron acetylacetonate is as attached Figure 4 shown.
Embodiment 3
[0061] Add 5 g of V to Reactor 1 2 o 3 , 35mL of cyclohexane and 35mL of acetylacetone, add 60mL of acetylacetone in the product collector 3, start magnetic stirring, feed 90mL / min of oxygen into the reactor 1, first heat the product collector 3 until the acetylacetone starts to reflux , then start to heat the reactor 1 to reflux, stop heating after reacting for 2 hours, place it in an ice bath after the product collector 3 is cooled, and precipitate blue crystals, suction filter, precipitate and dry to obtain blue acetylacetone Vanadyl, the yield is 87%. The purity of the obtained vanadyl acetylacetonate was determined to be 99% by chelate titration. The X-ray diffraction spectrogram of gained vanadyl acetylacetonate is as attached Figure 5 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com