Novel atenolol tablets and preparation method thereof
A tenolol tablet, a new type of technology, applied in the field of medicine, can solve the problems of poor oral absorption and low bioavailability, and achieve the effect of simple operation, good reproducibility, and easy mass production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
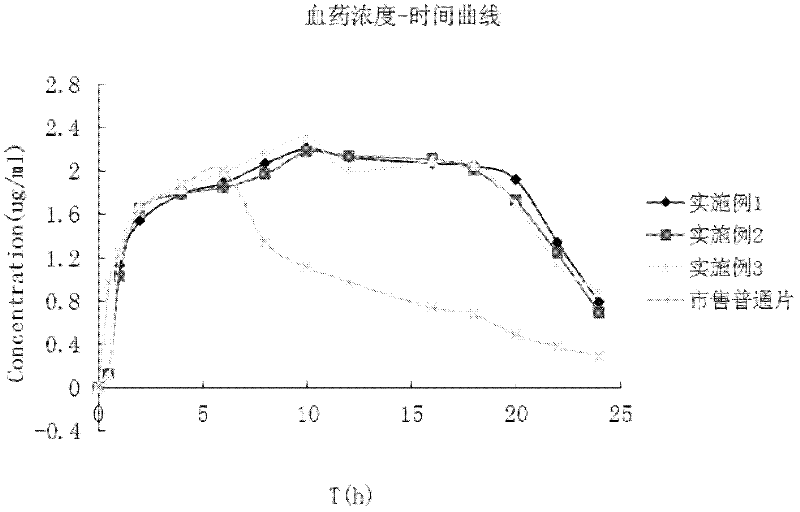
Image
Examples
Embodiment 1
[0025] A kind of novel atenolol tablet preparation comprises the component of following percentage by weight:
[0026]
[0027] The production method is as follows:
[0028] (1) Weigh the formula amount of atenolol and poloxamer, mix and pass through an 80-mesh sieve, and heat to melt in an 80°C water bath;
[0029] (2) Cool and solidify the melt obtained in step (1) rapidly at -20°C in an ice-salt bath, take it out after 1 hour, and dry it in a desiccator for 48 hours;
[0030] (3) pulverize the dry matter obtained in step (2) and cross an 80 mesh sieve to obtain atenolol solid dispersion;
[0031] (5) The dispersion obtained in step (3) is mixed with carmellose, cetyl alcohol, magnesium carbonate, and magnesium stearate in the prescribed amount and then directly compressed into tablets to obtain floating tablets retained in the stomach of atenolol .
Embodiment 2
[0033] A kind of novel atenolol tablet preparation comprises the component of following percentage by weight:
[0034]
[0035]
[0036] The production method is as follows:
[0037] (1) Weigh the formula amount of atenolol and poloxamer, mix and pass through an 80-mesh sieve, and heat to melt in an 80°C water bath;
[0038] (2) Cool and solidify the melt obtained in step (1) rapidly at -20°C in an ice-salt bath, take it out after 1 hour, and dry it in a desiccator for 48 hours;
[0039] (3) pulverize the dry matter obtained in step (2) and cross an 80 mesh sieve to obtain atenolol solid dispersion;
[0040] (5) The dispersion obtained in step (3) is mixed with polyvinyl alcohol, stearyl alcohol, magnesium carbonate, and magnesium stearate in the prescribed amount, and then directly compressed into tablets to prepare atenolol gastric retention floating tablets.
Embodiment 3
[0042] A kind of novel atenolol tablet preparation comprises the component of following percentage by weight:
[0043]
[0044] The production method is as follows:
[0045] (1) Weigh the formula amount of atenolol and polyethylene glycol 6000, mix them evenly, pass through an 80-mesh sieve, and heat in a water bath at 80°C until melting;
[0046] (2) Cool and solidify the melt obtained in step (1) rapidly at -20°C in an ice-salt bath, take it out after 1 hour, and dry it in a desiccator for 48 hours;
[0047] (3) pulverize the dry matter obtained in step (2) and cross an 80 mesh sieve to obtain atenolol solid dispersion;
[0048] (5) The dispersion obtained in step (3) is mixed with the formula amount of carmellose, stearyl alcohol, magnesium carbonate, and micropowder silica gel, and then directly compressed into tablets to prepare atenolol gastric retention floating tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com