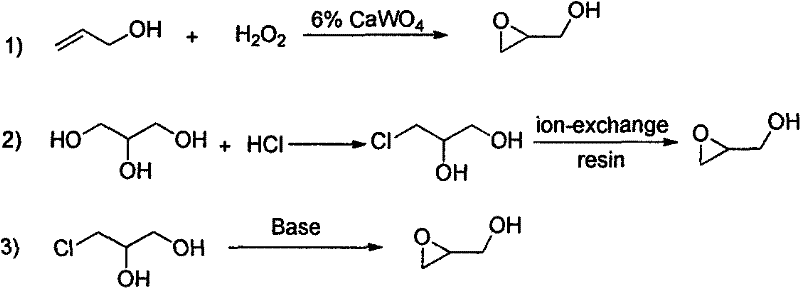
Method for synthesizing achiral glycidol
A glycidol and achiral technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problems not mentioned in the synthesis and application of achiral Salen catalysts, etc. Achieve the effect of no optical activity, easy operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Add 4.0g (0.035mol) trans-1,2-cyclohexanediamine, 16.8g (0.071mol) 2-hydroxy-3,5-di-tert-butylbenzaldehyde and 600mL methanol into a 2L four-necked flask , heated to 60°C, vigorously stirred and reacted for 3 hours, cooled to 40°C, added 17.0g (0.068mol) cobalt acetate tetrahydrate, heated to 60°C and stirred for 2 hours, then added 4.2g (0.070mol) to the reaction system mol) acetic acid, and oxidized by passing air into it, stirred and reacted at 60°C for 1 hour, concentrated by distillation, added 150mL of water, cooled to 10°C, stirred and crystallized, filtered, and the solid was washed with water to obtain 18.1g (0.027mol) of Salen-Co catalyst with a yield of 78%.
[0037] (2) Add 1.9g (0.0028mol) of achiral Salen-Co catalyst to 130g (1.4mol) of racemic epichlorohydrin, cool to 10°C, add 38g (1.5mol) of water dropwise, and maintain at React at 10-20°C for 20 hours. After completion of the reaction, add 0.5 g vitamin C, stir for 30 minutes, filter, and carry o...
Embodiment 2
[0040] (1) Mix 5.7g (0.050mol) of 1,2-cyclohexanediamine (trans:cis=70:30), 17.8g (0.10mol) of 2-hydroxyl-4-tert-butylbenzaldehyde Add 700mL of toluene into a 2L four-necked flask, heat to 60°C, stir vigorously for 3 hours, cool to 40°C, add 17.0g (0.068mol) cobalt acetate tetrahydrate, heat to 60°C and stir for 2 hours, Concentrate by distillation, add 150 mL of water, cool to 10°C, stir to crystallize, filter, and wash the solid with water to obtain 15.6 g (0.028 mol) of achiral Salen-Co catalyst precursor with a yield of 57%.
[0041] (2) Add 1.8g (0.0028mol) of achiral Salen-Co catalyst precursor to 130g (1.4mol) of racemic epichlorohydrin, add 0.3mL of acetic acid, oxidize with air for 30 minutes, and cool to At 5°C, 45g (2.5mol) of water was added dropwise, and the reaction was maintained at 5-10°C for 12 hours. After completion of the reaction, add 0.5 g vitamin C, stir for 30 minutes, filter, and carry out vacuum distillation on the filtrate, collect 80-84 ° C / 1 mmHg ...
Embodiment 3
[0043] (1) Add 3.0g (0.050mol) of 1,2-ethylenediamine, 16.8g (0.071mol) of 2-hydroxy-3,5-di-tert-butylbenzaldehyde and 500mL of ethanol into a 1L four-necked flask, heat to 60°C, vigorously stirred and reacted for 3 hours, cooled to 40°C, added 16.7g (0.068mol) manganese acetate tetrahydrate, heated to 60°C, stirred and reacted for 2 hours, concentrated by distillation, added 150mL of water, cooled to 10°C, Stir to crystallize, filter, and wash the solid with water to obtain 27.7 g (0.042 mol) of achiral Salen-Mn catalyst precursor with a yield of 84%.
[0044] (2) Join 1.8g (0.0028mol) achiral Salen-Mn catalyst precursor into 130g (1.4mol) racemized epichlorohydrin, add 0.3mL acetic acid, oxidize with air for 30 minutes, cool to At 5°C, 45g (2.5mol) of water was added dropwise, and the reaction was maintained at 5-10°C for 12 hours. After completion of the reaction, add 0.5 g vitamin C, stir for 30 minutes, filter, and carry out vacuum distillation on the filtrate, collect 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com