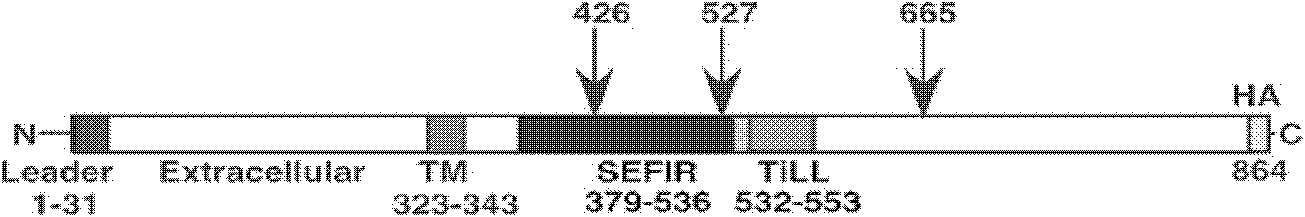
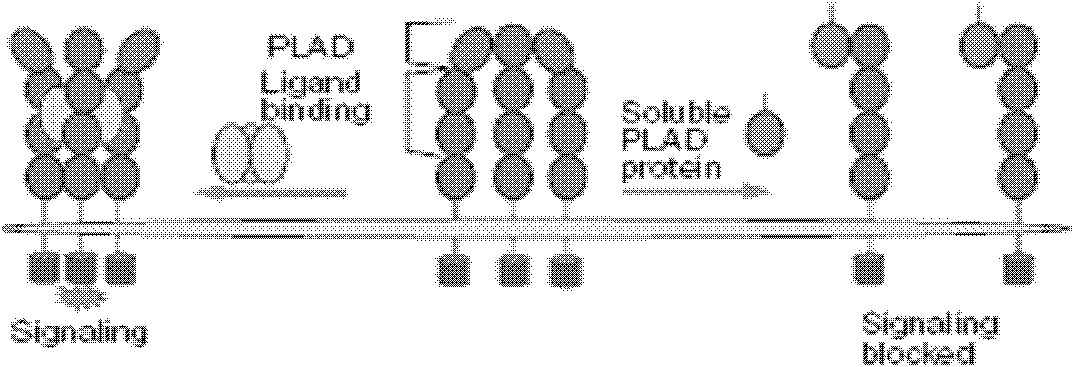Interleukin-17 receptor antagonist and application thereof in preparing anti-myocardial fibrosis medicine
A receptor blocker, interleukin technology, applied in the field of biomedicine, can solve the problems of large systemic side effects, unstable C-terminal of peptides, and limited drug research and development, achieving strong specificity and inhibiting receptor oligomerization. , the effect of high blocking efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1I
[0049] Embodiment 1 IL-17RA PLAD-IgG lentiviral vector construction
[0050] The construction and packaging of lentiviral vectors were assisted by Shanghai Yingweixin Biotechnology Co., Ltd.
[0051] 1. Experimental reagents and consumables
[0052] 1) Carrier: pLenO-DCE, the carrier map is as follows Figure 4 shown
[0053] 2) T4DNA ligase: NEB
[0054] 3) Restriction enzyme: NEB
[0055] 4) Gel recovery kit: Axygen
[0056] 5) PCR (enzymatic digestion) product purification kit: Axygen
[0057] 6) Thermosensitive phosphatase: NEB
[0058] 7) RNA extraction kit: Axygen
[0059] 8) MMLV First Strand cDNA Synthesis Kit: BBI
[0060] 9) DL2,000 DNA Marker: TaKaRa
[0061] 10) Electric constant temperature incubator: Shanghai Boxun Industrial Co., Ltd.
[0062] 11) Constant temperature shaker: Shanghai Sukun Industrial Co., Ltd.
[0063] 12) Electric constant temperature water bath: Shanghai Kanglu Experimental Instrument Co., Ltd.
[0064] 13) Electrophoresis instrum...
Embodiment 2I
[0150] Example 2 The effect of IL-17RA PLAD-Ig in anti-myocardial fibrosis
[0151] 1. Establishment of myosin-induced myocarditis model in rats
[0152] Materials: 6-week-old male Lewis rats were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.; purified porcine cardiac myosin was purchased from sigma company.
[0153] Method: 0.01M PBS and complete Freund's adjuvant added with 10 mg / ml Mycobacterium tuberculosis H37RA (Mycobacterium tuberculosis H37RA) were mixed according to the volume ratio of 1:1, and the mixed solution dissolved the purified porcine cardiac myosin ( Porcine cardiac myosin was purchased from Sigma-Aldrich, St Louis, MO, USA) to obtain immune emulsion for immunization of rats. Each rat was injected with 0.2 ml of the above-prepared emulsion from the footpad on day 0 and day 7 by a single subcutaneous injection. The rats after immunization were divided into two groups: IL-17RA PLAD-Ig group (n=13), on the 7th day, the ultrason...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com