(1,2,4)-triazolo-(4,3-b) (1,2,4)-triazine compounds, as well as preparation method and use thereof
A compound, triazolo technology, applied in the field of [1,2,4]triazolo[4,3-b][1,2,4]triazine compounds, their preparation and application, to achieve easy raw materials obtained, the reaction conditions are mild, and the raw materials are abundant
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
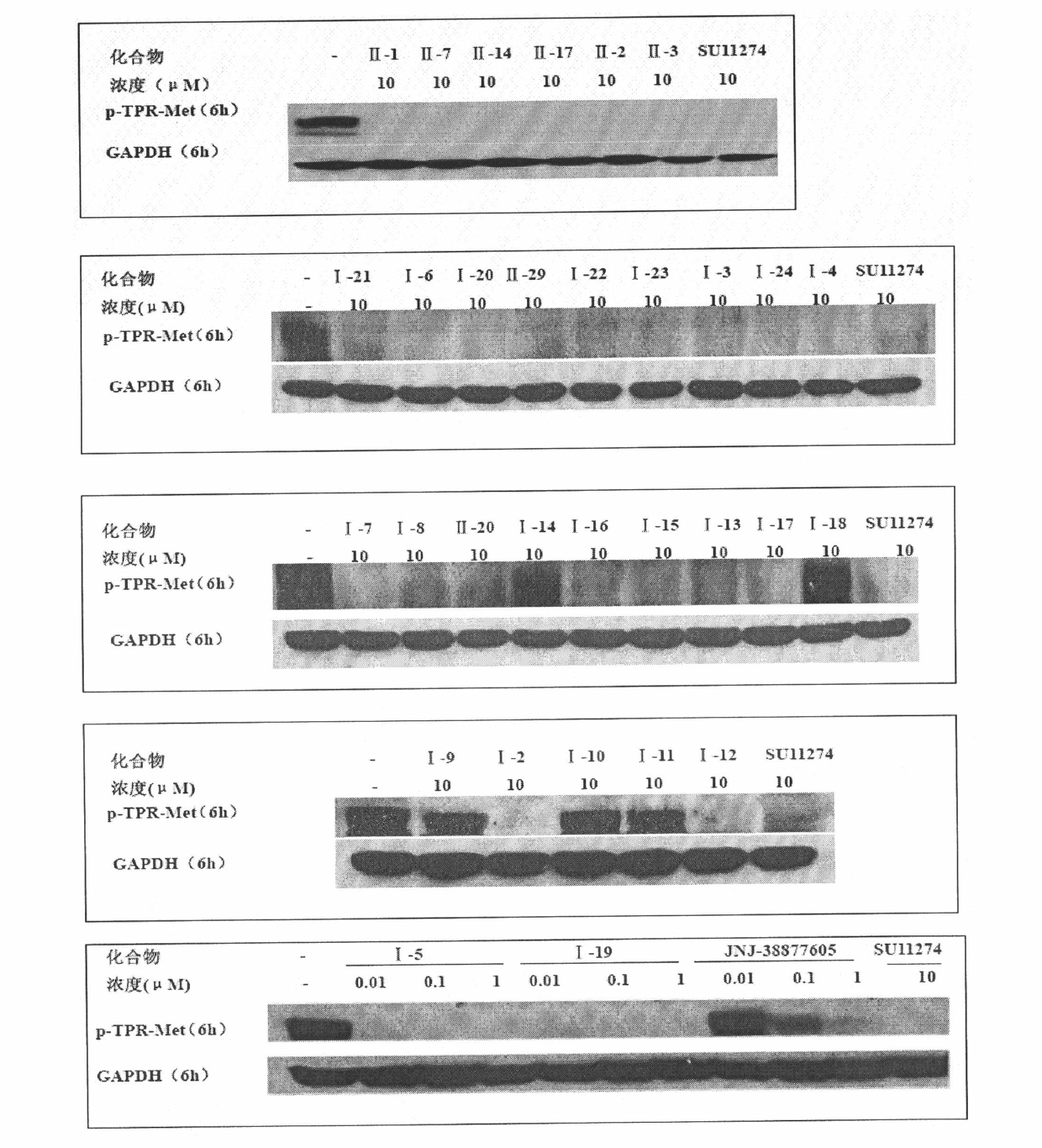
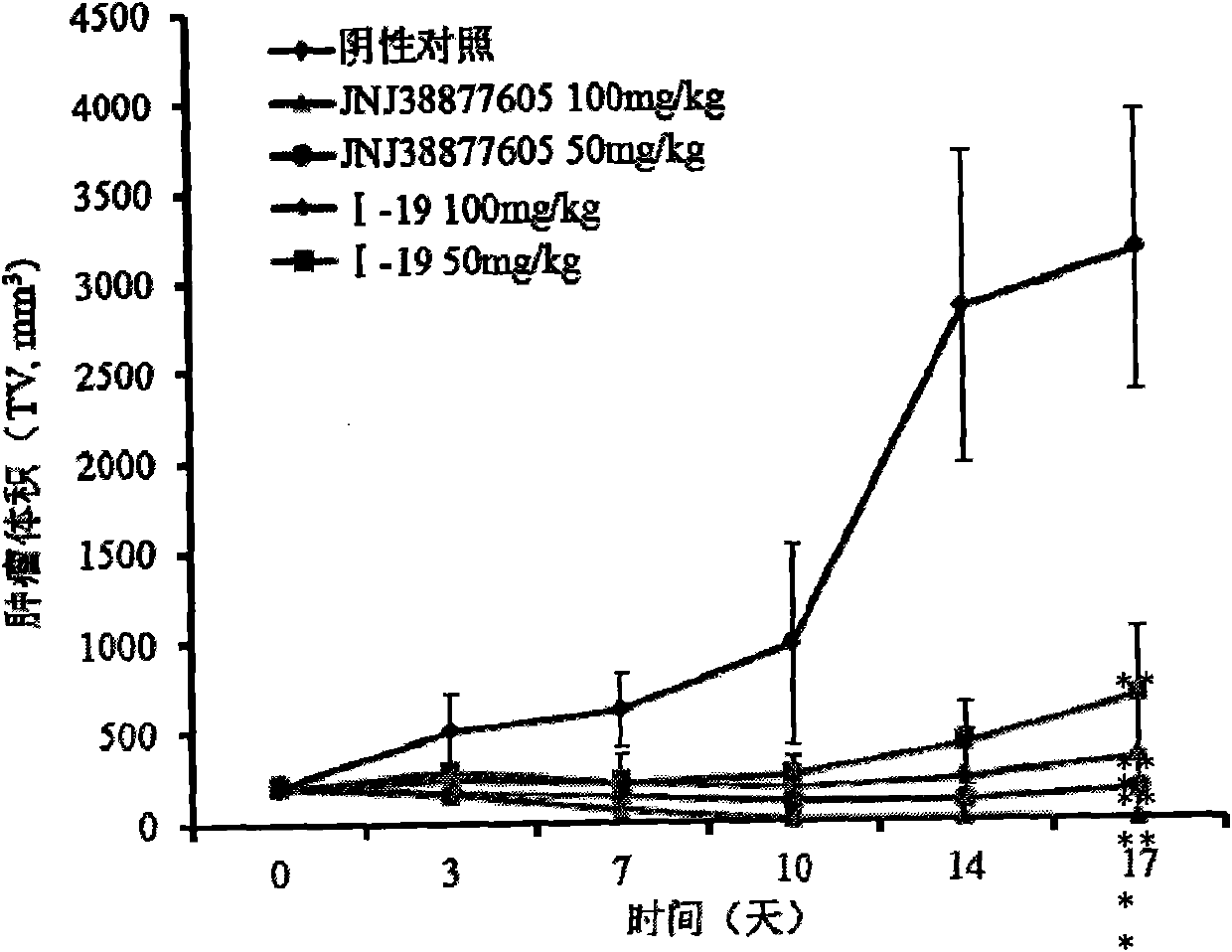
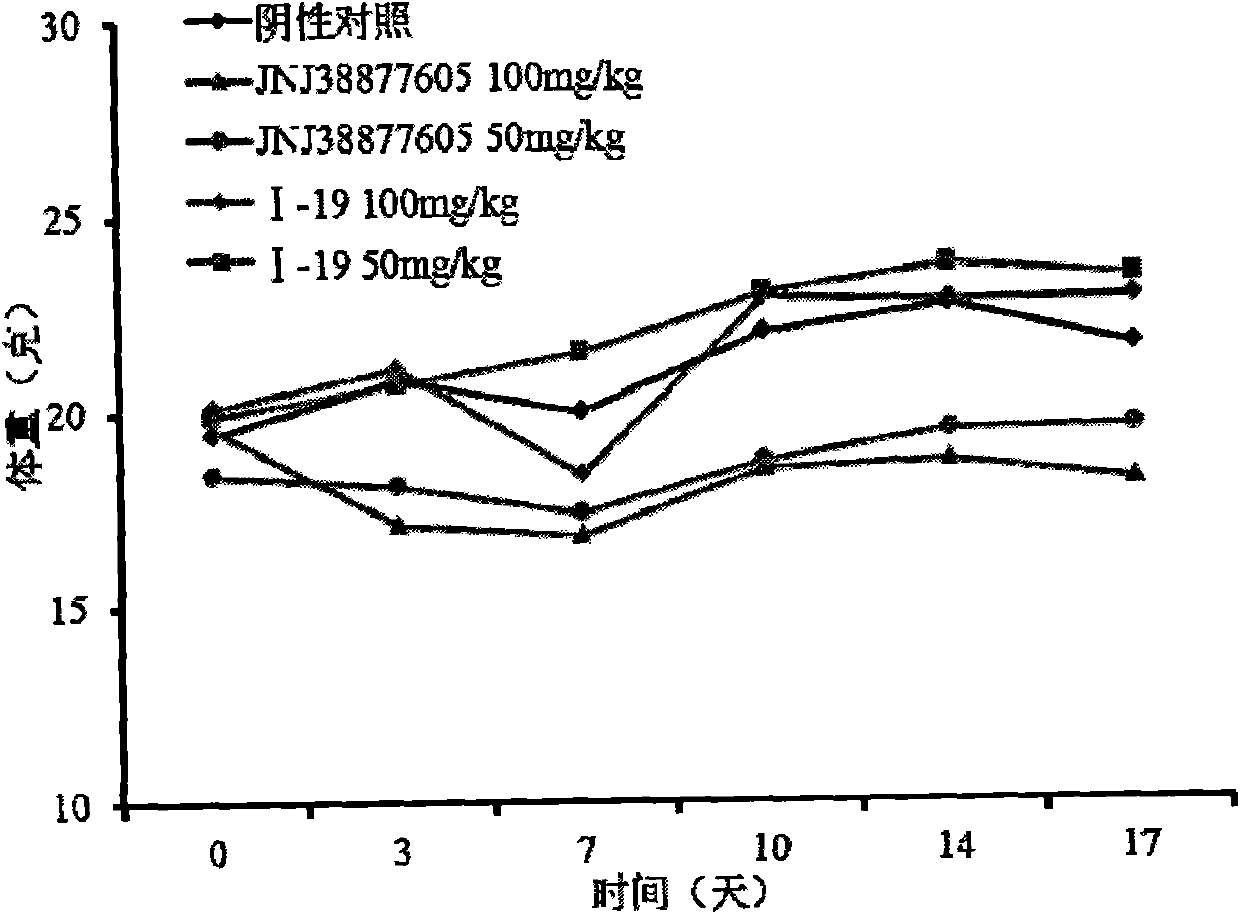
Image
Examples
Embodiment 1
[0064] 3-(6-Quinolinylmethyl)-6-phenyl[1,2,4]triazolo[4,3-b][1,2,4]triazine (I-1)
[0065]
[0066] Step 1: 2-oxo-2-phenylacetaldehyde (Ⅲ-1)
[0067] Add 14 grams of selenium dioxide, 62 milliliters of dioxane and 2.5 milliliters of water into a 100 milliliter round bottom flask, heat and stir at 50° C. until the selenium dioxide dissolves, add 15 grams of acetophenone, and cool the reaction solution after reflux for 4 hours. After filtration, the solvent was removed under reduced pressure, and the residue was subjected to column chromatography (ethyl acetate:petroleum ether=1:5) to obtain a white solid product III-1, weighing 9.8 g, with a yield of 58.5%. Mp 120-122°C. 1 H-NMR (300Hz, CDCl 3 -d 6 )δ: 9.67 (s, 1H), 8.04-8.15 (m, 2H), 7.53-7.66 (m, 3H).
[0068] Step 2: 2,2-diethoxyacetophenone (IV-1)
[0069] Dissolve 5.58 g of 2-oxo-2-phenylacetaldehyde (Ⅲ-1), 12.34 g of triethyl orthoformate, and 400 mg of p-toluenesulfonic acid in 40 ml of dichloromethane, stir and ...
Embodiment 2
[0082] 3-(6-quinolinemethyl)-6-(4-benzyloxyphenyl)[1,2,4]triazolo[4,3-b][1,2,4]triazine (Ⅰ -2)
[0083]
[0084] Step 1: 2-oxo-2-(4-benzyloxyphenyl)acetaldehyde (Ⅲ-2)
[0085] Dissolve 21.82 g of 4-benzyloxyacetophenone in 200 ml of DMSO, slowly drop into 33 ml of 48% hydrobromic acid with stirring at room temperature, heat and stir at 70°C for 8 hours after dropping, cool the reaction solution, and pour it into 200 ml Suction filter in ice water, wash the filter cake with water, and dry to obtain the white solid product III-2, weighing 21.47 g, with a yield of 92.7%. 1 H-NMR (300Hz, DMSO-d 6 )δ: 8.05(d, J=9.0Hz, 2H), 7.34-7.48(m, 5H), 7.13(d, J=9.0Hz, 2H), 5.22(s, 2H).
[0086] Step 2: 2,2-diethoxy-1-(4-benzyloxyphenyl)ethanone (IV-2)
[0087] Replace 2-oxo-2-phenylacetaldehyde (Ⅲ-1) with 2-oxo-2-(4-benzyloxyphenyl)acetaldehyde (Ⅲ-2), and the remaining raw materials, reagents and preparation The method is the same as step 2 in Example 1 to obtain pale yellow oily liq...
Embodiment 3
[0099] 3-(6-quinolinemethyl)-6-(2-thienyl)[1,2,4]triazolo[4,3-b][1,2,4]triazine (I-3)
[0100]
[0101] Step 1: 2-oxo-2-(2-thienyl)acetaldehyde (Ⅲ-3)
[0102] Acetophenone is replaced with 2-thienyl ethyl ketone, and all the other required raw materials, reagents and preparation methods are the same as step 1 in Example 1 to obtain yellow solid product 2-oxo-2-(2-thienyl)acetaldehyde ( III-3).
[0103] Step 2: 2,2-diethoxy-1-(2-thienyl)ethanone (IV-3)
[0104] Replace 2-oxo-2-phenylacetaldehyde (Ⅲ-1) with 2-oxo-2-(2-thienyl)acetaldehyde (Ⅲ-3), and the rest of the required raw materials, reagents and preparation methods are implemented in the same way Step 2 in Example 1 gave the product IV-3 as a pale yellow oily liquid. 1 H-NMR (300Hz, CDCl 3 -d 6 )δ: 8.06(d, J=5.4Hz, 1H), 7.66(dd, J=0.9, 4.8Hz, 1H), 7.14(m, 1H), 5.12(s, 1H), 3.63-3.78(m, 4H ), 1.23-1.28(m, 6H).
[0105] Step 3: 3-Methylthio-6-(2-thienyl)-1,2,4-triazine (V-3)
[0106] Replace 2,2-diethoxyacetophen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com