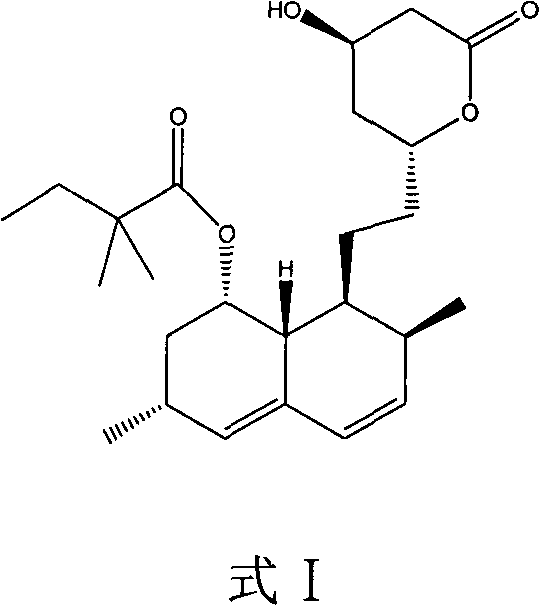
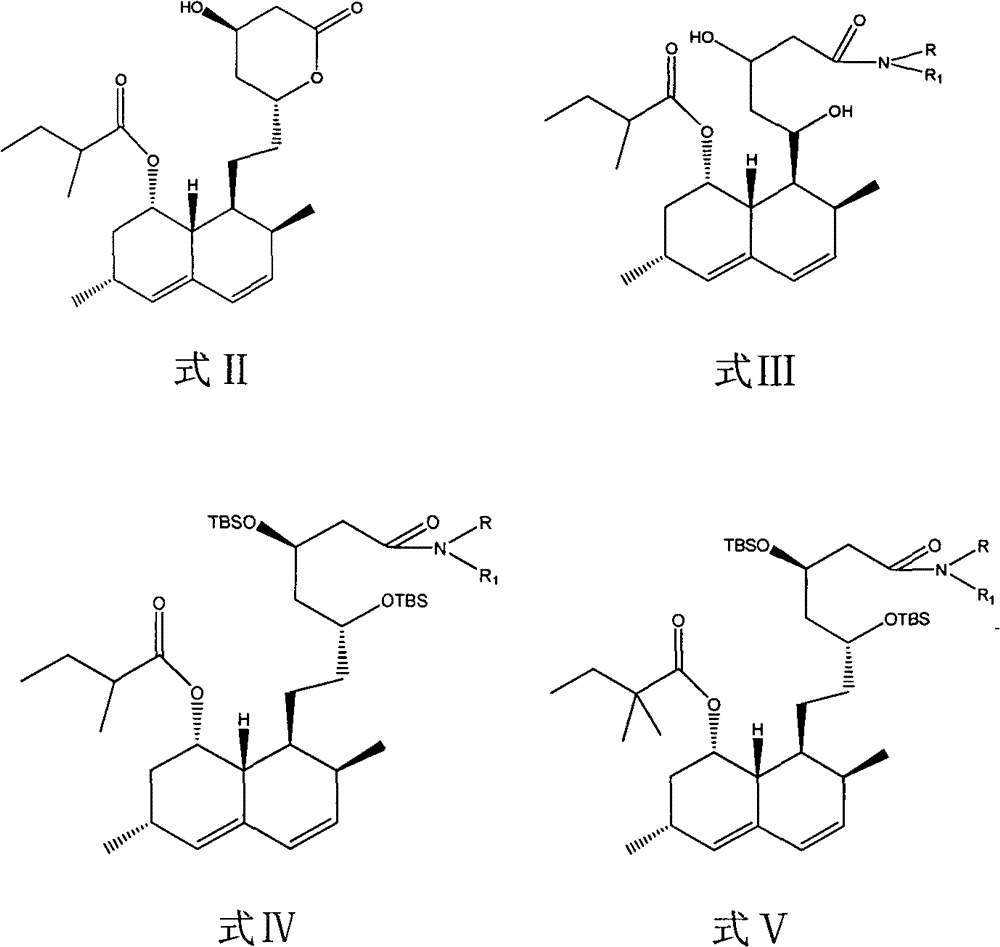
Dihydroxyl protection method of lovaamide and preparation method of simvastatin
A technology of lovastatin and hydroxyl protecting agent, which is applied in the field of simvastatin intermediate and simvastatin preparation, can solve the problems of increasing production cost, polluting the environment, inconvenient recycling of dimethylformamide, etc., and achieve reduction in production costs, effects of avoiding negative impacts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Add 84ml of n-butylamine to 100g of lovastatin, heat up to 80°C under stirring, reflux for 1-1.5 hours, cool down to 50°C, recover excess n-butylamine under reduced pressure (put it into the next batch for use), and obtain lovastatin for direct use. react in the next step.
[0048] Add 150ml of tetrahydrofuran to dissolve the lovaamide prepared by the above reaction, add 42g of imidazole, 86g of tert-butyldimethylsilyl chloride, and 2g of dimethylaminopyridine, heat to reflux, and TLC detects that the reactant completely disappears. Cool, filter, and wash the filter cake with 50ml of tetrahydrofuran, suck it dry, and recover imidazole. The filtrate was concentrated to dryness, and the recovered solvent was applied mechanically. After the residue was dissolved in 500ml cyclohexane, the filtrate was dissolved with 500ml of 1% NH 3 Wash twice with water, then wash with 500ml water, separate the organic layer, wash with anhydrous Na 2 SO4 Dry for 4 hours, filter, and conc...
Embodiment 2
[0050] Dissolve the lovaamide prepared by the method in Example 1 in 150 ml of acetonitrile, add 42 g of imidazole, 86 g of tert-butyldimethylsilyl chloride, and 5 g of potassium iodide, and heat to 75° C. to react, and TLC detects that the reactant completely disappears. Cool, filter, wash the filter cake with 50ml of acetonitrile, pump dry to recover imidazole, concentrate the filtrate to dryness, recover the acetonitrile for mechanical use, add 500ml of cyclohexane to dissolve the residue, and dissolve the filtrate with 500ml of 1% NH 3 Wash twice with water, then wash with 500ml water, separate the organic layer, wash with anhydrous Na 2 SO 4 Dry for 4 hours, filter, and concentrate the filtrate to dryness to obtain 176.5 g of the product lovaamide disiloxane, which is directly used in subsequent reactions.
Embodiment 3
[0052] Dissolve the lovaamide prepared by the method in Example 1 in 200ml of methylation recovery solvent (containing tetrahydrofuran, cyclohexane and n-hexane), add imidazole 42g, tert-butyldimethylsilyl chloride 86g, potassium iodide 5g, dimethyl Aminopyridine 2g, heat up to reflux with stirring, keep warm for reaction, TLC detects that the reactant completely disappears, cool until the insoluble matter is completely precipitated, remove the insoluble matter, and the filtrate is directly used for the methylation reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com