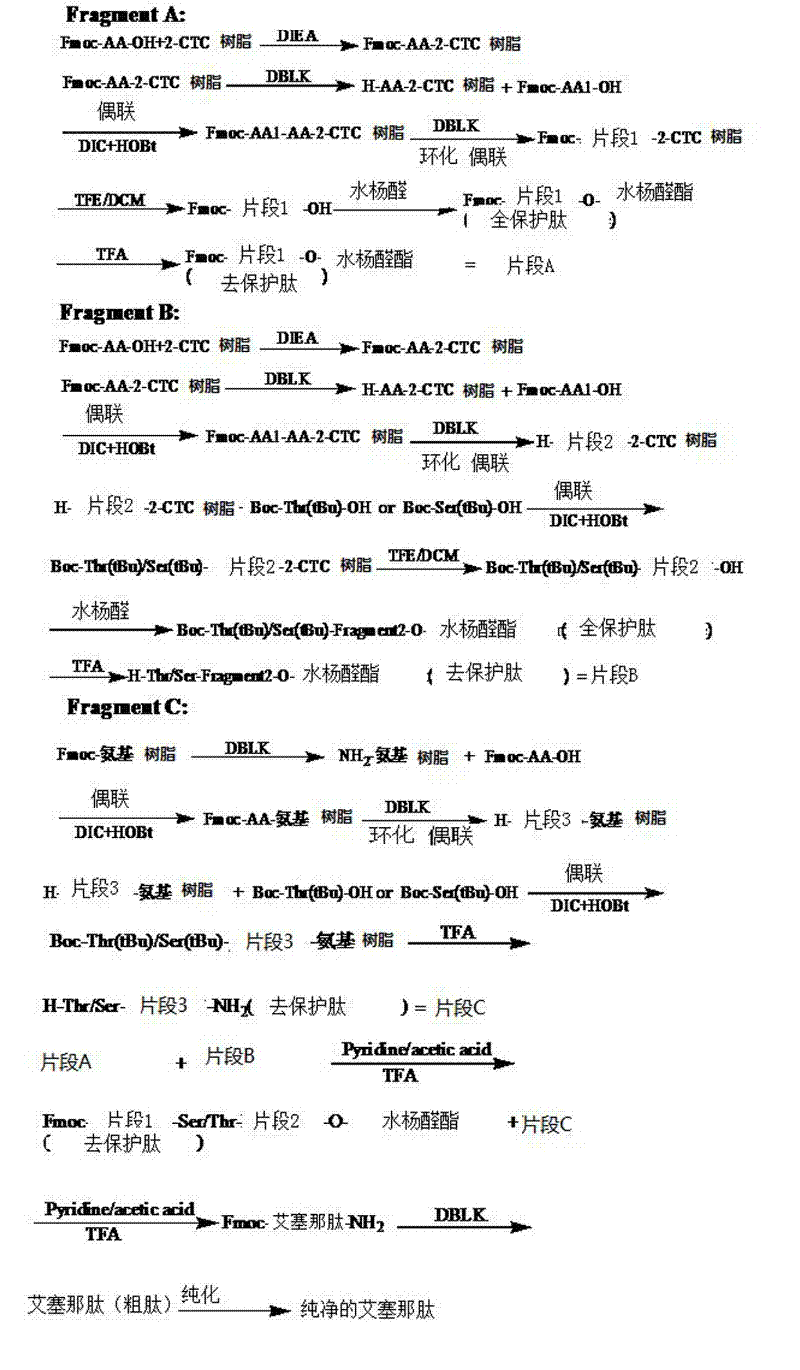
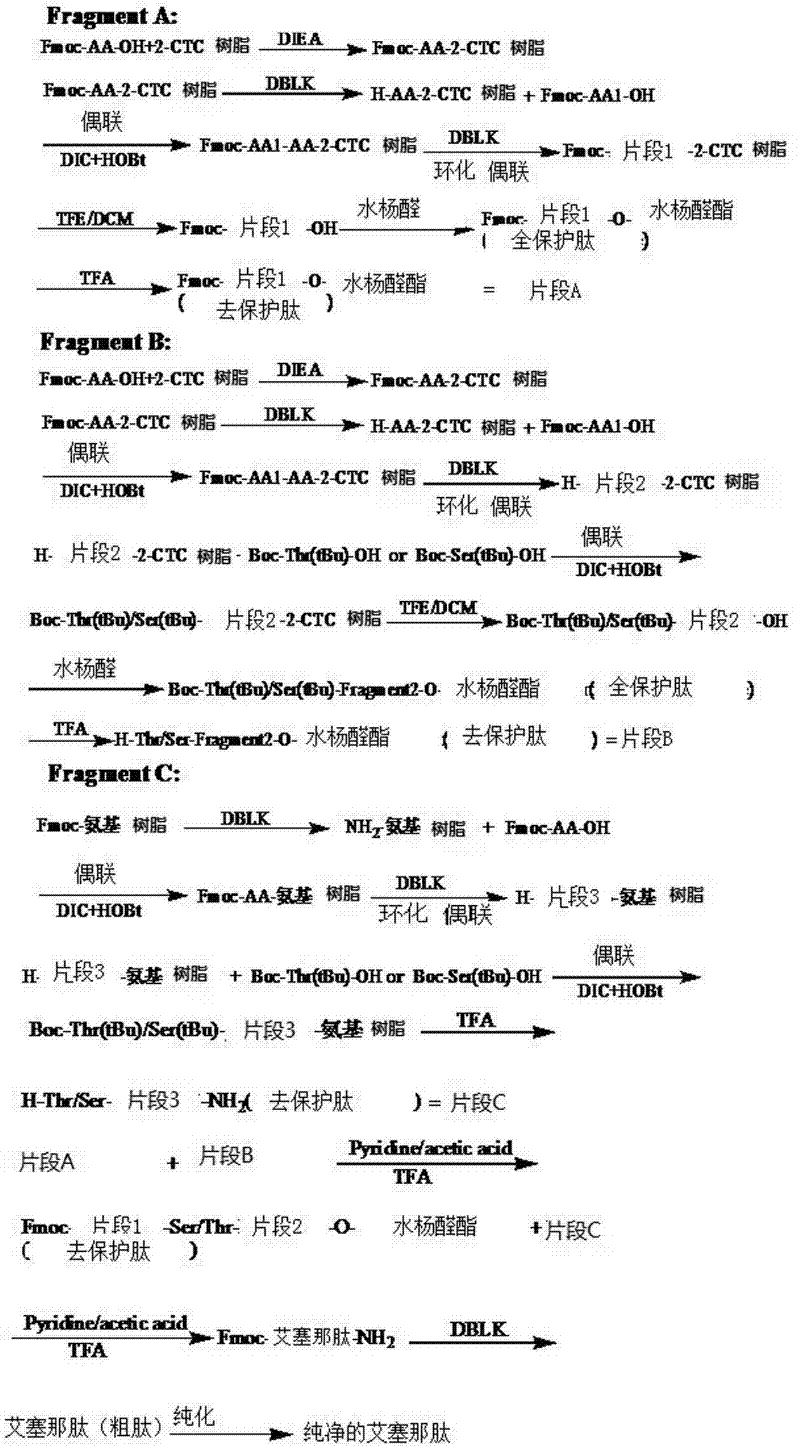
Method for preparing exenatide with natural coupling method
An exenatide and coupling technology, which is applied in the field of preparation of polypeptide drugs, can solve the problems of unfavorable large-scale production, strong hydrophobicity, long synthesis cycle, etc., and achieves advantages of large-scale production, easy post-processing, and short synthesis cycle. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of fully protected peptide fragment 11 with Fmoc protection at the N-terminus
[0049] SEQ ID No.11, also known as peptide fragment 11 in the present invention, its molecular structure is His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-OH; fully protected peptide fragment 11 refers to The peptide fragment 11 protected by the protecting group tBu and OtBu has the same meaning as "Exenatide (1-10)" in the present invention.
[0050] A. Preparation of Fmoc-Leu-CTC resin
[0051] Weigh 20 g of 2-CTC resin with a substitution degree of 0.5 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes; take 7.1 g of Fmoc-Leu-OH and dissolve it in DMF, ice Add 5.22mL DIEA to activate in a water bath; then add to the above-mentioned reaction column filled with resin, react for 2 hours; add 8mL of anhydrous methanol to block for 1 hour. Wash 3 times with DMF and 3 times with DCM, seal with anhydrous methanol...
Embodiment 2
[0056] Example 2: Preparation of Fmoc-Exenatide (1-10)-O-salicylaldehyde ester
[0057] Fmoc-Exenatide (1-10)-OH crude peptide (10.9g, 6mmol), salicylaldehyde (3.1ml, 30mmol) and DCC (2.5g, 12mmol) were dissolved in 400ml DCM, and reacted at room temperature for 2h. After the reaction was completed, the volume of the reaction solution was rotary evaporated to a volume ratio of <25%, and then added to 100 times the amount of anhydrous ether, centrifuged, washed with anhydrous ether, and vacuum-dried to obtain Fmoc-Exenatide (1-10)- O-salicylaldehyde ester 10.73g.
Embodiment 3
[0058] Embodiment 3: Remove Fmoc-exenatide (1-10)-O-salicylaldehyde ester protecting group
[0059] Fmoc-exenatide (1-10)-O-salicylaldehyde ester (9.6g, 5mmol) was placed in the reaction vessel, added in 200ml lysate (TFA:H 2 O:TIS volume ratio=95:2.5:2.5), stirred at room temperature for 2h. After the reaction, the reaction solution was poured into 2L of anhydrous ether, centrifuged, washed with anhydrous ether, and vacuum-dried to obtain the Fmoc-peptide fragment 11-O-salicylaldehyde ester, namely Fmoc-His-Gly-Glu-Gly -Thr-Phe-Thr-Ser-Asp-Leu-O-salicylaldehyde ester, product weight 6.85g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com